Design of Structured Adhesion MiniproteinsResearch - Florian Häge |
 |
|
|
|
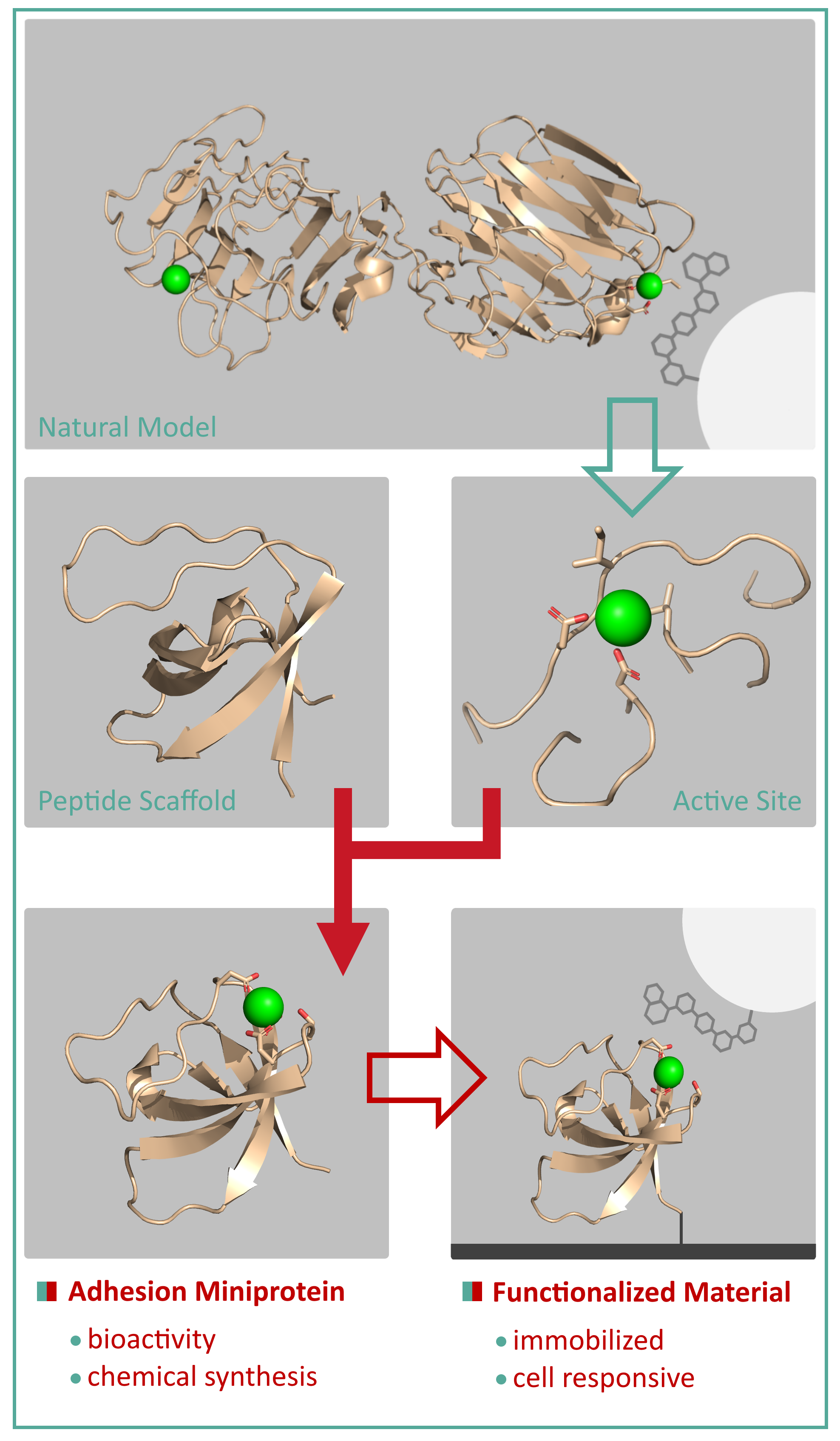
Tissue Engineering requires artificial constructs to support the formation of tissue from cells. These support structures consist of microporous organic or inorganic material that immobilizes the cells of interest. There are two common strategies to functionalize materials for tissue engineering: the immobilization of cell adhesion motifs, which bind to cell receptor, or the immobilization of entire domains of cell binding proteins. However, the small motifs lack a defined three-dimensional structure and thus bioactivity and the large protein fragments are difficult to immobilize and often denaturate in the attempt. My project aims to unify the bioactivity of a cell binding protein with the chemical modifiability of cell adhesion motifs: the design of adhesion miniproteins based on independently folding peptide scaffolds with a cell binding active site. Extracellular matrix proteins are known to bind the carbohydrate chains of cell receptors with, often calcium-dependent, protein-carbohydrate binding site. These active sites, found in LG domains, calmodulin or lectins, serve as ideal natural models for the peptide design. The active site of a natural model protein is incorporated into the sequence of a β-sheet peptide scaffold, such as the SH3 or WW domains, using computational modeling to ensure similar-to-native folding. Suitable sequences are then synthesized by solid-phase peptide synthesis (SPPS) and characterized with respect to structure and binding. A sufficiently biomimetic miniprotein is planned to be immobilized on materials to add cell responsiveness and thus generate a functionalized material for retina tissue engineering. |


