Research AK Bunz: Large Heteroacenes
| Pentacene and TIPS-pentacene are nowadays reference materials for thin-film transistors (OFETs) exhibiting excellent hole mobilities but poor electron transport characteristics. Introduction of electronegative atoms directly into the perimeter of the acenes preserves brick-wall-like packing for TIPS-tetraazapentacene (TIPS-TAP) with electron mobilities up to 3.3 cm2V-1s-1. |
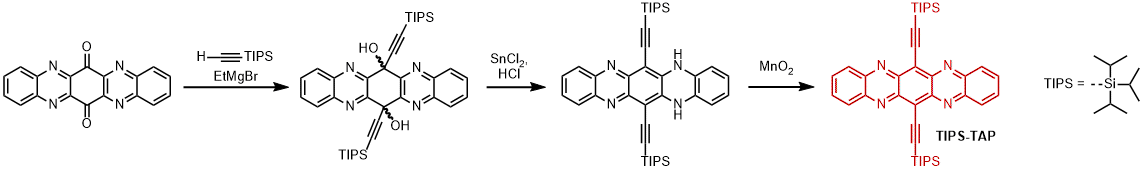 |
| Azapentacenes can be accessed via three different routes: 1) Condensations of 1,2-diarylenediamines with ortho-quinones, 2) addition-elimination reactions of arylenediamines with electron-deficition arenes or 3) Buchwald-Hartwig couplings. In order to obtain tetraazapentacenes employing classic condensation reactions, a reduction protocol to the elusive bisalkynylated 3,6-diiminocyclohexa-1,4-diene-1,4-diamine has been developed starting from TIPS-DBT (left). This building block accesses novel derivatives in a one-step condensation reaction with ortho-quinones. Alternatively, benzothiadiazoles are employed as masked arylenediamines. Reductive ring opening (Zn or SmI2) furnishes acenylenediamines for subsequent condensation reactions. Fourfold bromination (Br4-TIPS-TAP) increases the electron mobility of TIPS-TAP by a factor of 50 under identical processing conditions. Moreover, Br4-TIPS-TAP is so electron-poor that its radical anion withstands re-oxidation in dry air. |
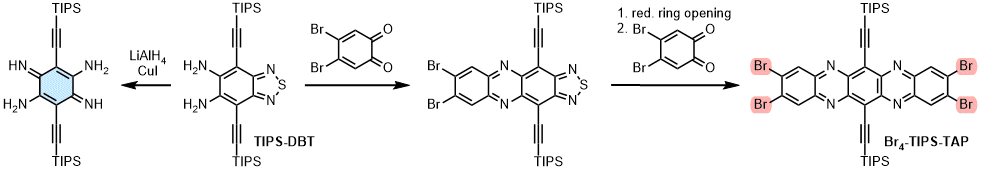 |
| Synthesis of TAP-derivatives may also proceed via addition-elimination routes. Extension of this concept by coupling to larger fluorinated arenes results in dimerization after oxidation. |
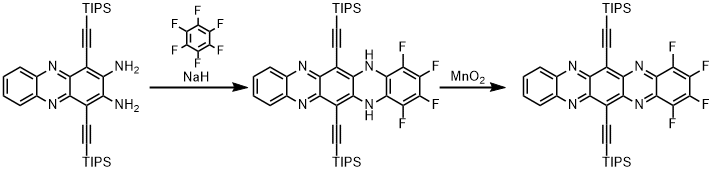 |
| The third method, the Pd-catalyzed Buchwald-Hartwig coupling reactions, accesses a variety of azahexacenes under relatively mild conditions. Both activated (electron-poor) as well as deactivated 1,2-dihaloarenes can be employed. |
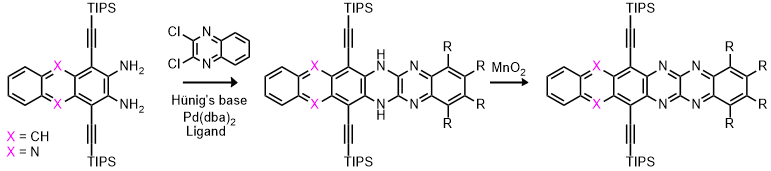 |
| Similar to acenes, increasing azaacenes in size renders them more reactive with respect to [4+4] dimerizations concomitant with the restoration of Clar sextets as the driving force for this reaction. The bulkier the silyl substituent, the slower the dimerization process: The persistency of diazaheptacenes increases in the order R = sBu > iPr. |
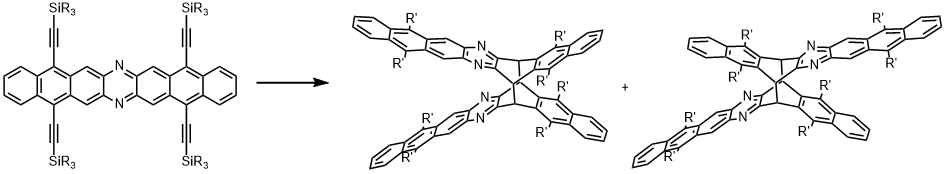 |
| The stabilization of the larger (aza)acene still is a challenge. Upon tetrafold benzannulation, the azaheptacene core is stabilized due to the presence of four additional Clar sextets. This concepts was also extended to a persistent nonacene. The optical properties of such species correspond to π-extended azapentacenes and heptacenes, respectively. |
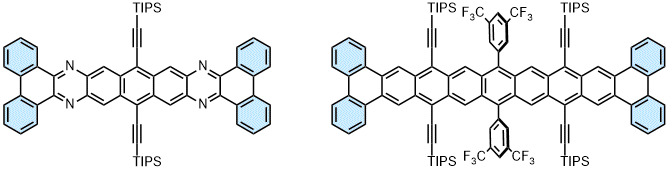 |
| An alternative strategy is 'clipping and jacketing' (aza)acenes covalently into a ring to serve as a protection of the (fairly) sensitive π-system complementing hitherto uncontested TIPS-ethynylation. Doubly bridged azapentacenes are, compared to their TIPS-ethynylated analogues, more stable with respect to photodegradation under inert and air atmosphere. In contrast, a pentacene derivative is more easily photooxidized. This concept is easily extendended to a tetraazahexacene. |
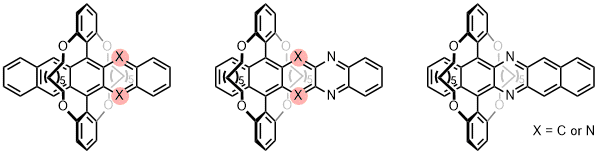 |
| Selected References |
| Miao, S.; Appleton, A. L.; Berger, N.; Barlow, S.; Marder, S. R.; Hardcastle, K. I.; Bunz, U. H. F.: "6,13-Diethynyl-5,7,12,14-tetraazapentacene". Chem. Eur. J. 2009, 15, 4990-4993. |
| P. Biegger, M. Schaffroth, K. Broedner, O. Tverskoy, F. Rominger, U. H. F. Bunz: "A bisalkynylated 3,6-diiminocyclohexa-1,4-diene-1,4-diamine". Chem. Comm. 2015, 51, 14844-14847. |
| H. Reiss, L. Ji, J. Han, S. Koser, O. Tverskoy, J. Freudenberg, F. Hinkel, M. Moos, A. Friedrich, I. Krummenacher, C. Lambert, H. Braunschweig, A. Dreuw, T. Marder, U. H. F. Bunz: "Bromination Improves Tetraazapentacene's Electron Mobilities". Angew. Chem. Int. Ed. 2018, 57, 9543-9547. |
| J. U. Engelhart, B. D. Lindner, O. Tverskoy, F. Rominger, U. H. F. Bunz: "Partially Fluorinated Tetraazaacenes by Nucleophilic Aromatic Substitution". J. Org. Chem. 2013, 78, 10832-10839. |
| B. D. Lindner, J. U. Engelhart, O. Tverskoy, A. L. Appleton, F. Rominger, A. Peters, H.-J. Himmel, U. H. F. Bunz: "Stable Hexacenes through Nitrogen Substitution". Angew. Chem. Int. Ed. 2011, 50, 8588-8591. |
| J. U. Engelhart, B. D. Lindner, M. Schaffroth, D. Schrempp, O. Tverskoy, U. H. Bunz: "Substituted Tetraaza- and Hexaazahexacenes and Their N,N'-Dihydro-Derivatives. Syntheses, Properties and Structures" Chem. Eur. J. 2015, 21, 8121-8129. |
| J. U. Engelhart, O. Tverskoy, U. H. F. Bunz: "A Persistent Diazaheptacene-Derivative". J. Am. Chem. Soc. 2014, 136, 15166-15169. |
| M. Müller, H. Reiss, O. Tverskoy, F. Rominger, J. Freudenberg, U. H. F. Bunz: "Stabilization by Benzannulation: Butterfly-Azaacenes". Chem. Eur. J. 2018, 24, 12801-12805. |
| M. Müller, S. Maier, O. Tverskoy, F. Rominger, J. Freudenberg, U. H. F. Bunz: "Tetrabenzononacene: "Butterfly Wings" Stabilize the Core". Angew. Chem. Int. Ed. 2020, 59, 1966-1969. |
| L. Ahrens, O. Tverskoy, S. Weigold, M. Ganschow, F. Rominger, J. Freudenberg, U. H. F. Bunz: "(Aza)Pentacenes Clipped into a Ring: Stabilization of Large (Aza)Acenes". Angew. Chem. Int. Ed. 2021, 60, 9270-9273. |
Webadmin:
E-Mail
Letzte Änderung:
27.08.2021



