Electrocoagulation to remove arsenic from groundwater - investigations on the procedure and geochemistry
|
Project team: Daniel Müller, Martin Maier, Charlotte Stirn |
|
Background/Summary
Groundwater is the most common source of drinking water worldwide. Accordingly, contaminated groundwater is one of the biggest health problems of today, especially in countries like Bangladesh and West Bengal where groundwater is naturally high in arsenic and usually not treated before consumption.
A large number of technical solutions exist for arsenic removal. However, these approaches often entail high costs, complex handling, or the use of additional chemicals. So far, this prevented the application of those technologies particularly in infrastructural and financially weak regions by now. Thus, there is a need for cost efficient, simple and robust methods for removing arsenic from drinking water. Electrocoagulation is one these so-called low-cost and low-tech procedures, because it is suitable for removing arsenic and other pollutants from water with simple iron plates and low electric power consumption.
The electrolytic process uses iron electrodes to generate in-situ iron (sacrificial anode) according to Faraday’s law. When iron complexes are formed, arsenic is also incorporated and can be removed by quickly gravitationally settling of flocs (Fig. 1).
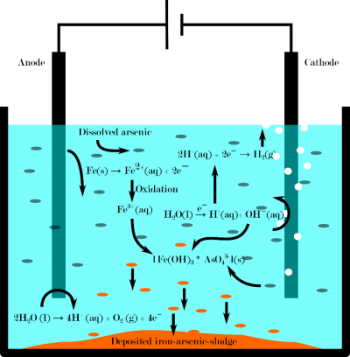
Figure 1: Relevant processes in electrocoagulation are shown. Various iron-arsenic-compounds can be formed (here representative: Fe(OH3) * AsO43-).
Laboratory and field experiments were carried out in batch mode and in continuous flow mode (Fig. 2 & 3). During the test’s geochemical parameters temperature, pH, electrical conductivity, oxygen content and redox potential were systematically monitored and water samples were taken at various timesteps to analyse water composition. Experiments were carried out in batch mode and in continuous flow mode (Fig. 2 & 3).
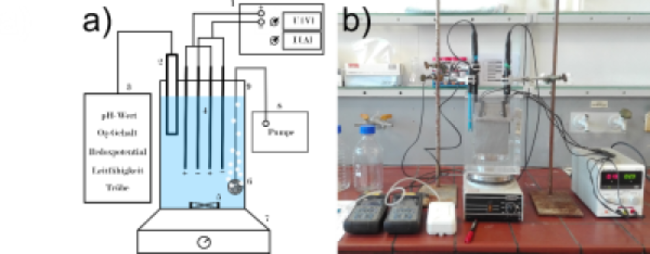
Figure 2: a) 1: DC-Power supply 2: Iron electrodes 3: Measuring device 4: Measuring probes 5: Aquarium air pump 6: Bubbles stone 7: Magnetic stirrer 8: Magnetic stir bar 9: Plexiglass EC-reactor b) Laboratory set up.
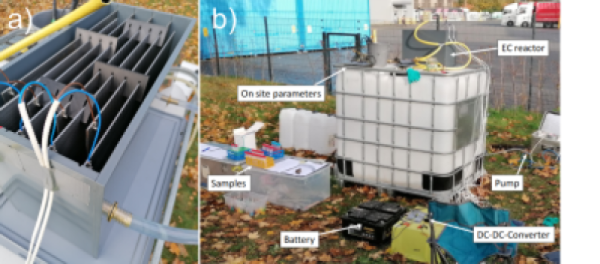
Figure 3: Field experiment set up of the continuous EC-reactor. a) Close up of the reactor b) Complete experimental setup.
The first promising results show a fast arsenic removal. Initial concentrations of about 500 µgAs/l are completely removed within less than one hour of electrocoagulation. Furthermore, all on-site parameters (e.g. pH) remain within an un-critical range. The geochemical setting of the groundwater changes negligibly, indicating that no harmful effects take place. Anyhow, ecotoxicological studies are needed. Latest field experiments show that electrocoagulation is an effective technique to remove high arsenic concentrations of up to 14400 µgAs/l (Fig. 4) as well.
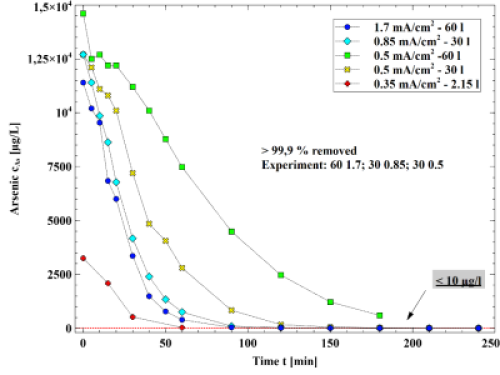
Figure 4: Arsenic concentration over the time of electrocoagulation. Latest field experiments were carried out with different current density, water volume and surface/volume ratio.
Overall electrocoagulation offers a cheap, robust method to remove arsenic which does not require a chemical supply chain. The energy required is low and the system works at safety extra-low voltage.