Publications - TP 2
Dietl, A. Wild, K. Simon, B. (2013). (1)H, (13)C, and (15)N chemical shift assignments of the phosphotyrosine binding domain 2 (PTB2) of human FE65. Biomol NMR Assign. Jan.12 EPUB, PMID 23315337
Mueller, U. Wild, K. (2013) . Structure and Function of the APP Intracellular Domain in Health and Disease. In Tech open http://dx.doi.org/10.5772/54543
Isbert S, Wagner K, Eggert S, Schweitzer A, Multhaup G, Weggen S, Kins S, Pietrzik CU. (2012) APP dimer formation is initiated in the endoplasmic reticulum and differs between APP isoforms. Cell Mol Life Sci. 69(8):1353-75.
Baumkötter, F., Wagner, K., Eggert, S., Wild, K., and Kins, S. (2012). Structural aspects and physiological consequences of APP/APLP trans-dimerization. Exp Brain Res 217(3-4), 389-395.
Riemer and Kins S. (2012): Axonal transport and mitochondrial dysfunction in Alzheimer’s Disease. Neurodeg Disease, in press
Mueller, U. and Wild, K. Structure and Function of the APP Intracellular Domain in Health and Disease. In Tech, Book chapter in preparation, provisionally accepted.
Klug, W., Dietl, A., Simon, B., Sinning, I., Wild, K. (2011). Phosphorylation of LRP1 regulates the interaction with Fe65. FEBS Lett 585, 3229-3235.
Bozkurt K, Stjepanovic G, Vilardi F, Amlacher S, Wild K, Bange G, Favaloro V, Rippe K, Hurt E, Dobberstein B, I. S (2009) Structural insights into tail-anchored protein binding and membrane insertion by Get3. Proc Natl Acad Sci U S A in press.
Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Müller U, Beyreuther K, Brady S, Morfini G, Kins S. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci. 2009 Nov 18;29(46):14534-44.
Stengel KF, Holdermann I, Cain P, Robinson C, Wild K, Sinning I (2008): Structural basis for specific substrate recognition by the chloroplast signal recognition particle protein cpSRP43. Science 321:253-256.
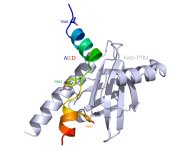
Radzimanowski J, Simon B, Sattler M, Beyreuther K, Sinning I & Wild K. (2008): Structure of the intracellular domain of the amyloid precursor protein in complex with Fe65-PTB2. EMBO Rep 9, 1134-1140.
Radzimanowski J, Ravaud S, Schlesinger S, Koch J, Beyreuther K, Sinning I & Wild K. (2008): Crystal structure of the human Fe65-PTB1 domain. J Biol Chem 283, 23113-23120 .
Radzimanowski J, Ravaud S, Beyreuther K, Sinning I. & Wild K. (2008): Mercury-induced crystallization and SAD phasing of the human Fe65-PTB1 domain. Acta Crystallogr Sect F Struct Biol Cryst Commun 64, 382-385.
Radzimanowski J, Beyreuther K, Sinning I & Wild K. (2008): Overproduction, purification, crystallization and preliminary X-ray analysis of human Fe65-PTB2 in complex with the amyloid precursor protein intracellular domain. Acta Crystallogr Sect F Struct Biol Cryst Commun 64, 409-412.
Stengel KF, Holdermann I, Cain P, Robinson C, Wild K, Sinning I. Structural basis for specific substrate recognition by the chloroplast signal recognition particle protein cpSRP43. Science. 2008 Jul 11;321(5886):253-6.
Back S, Haas P, Tschape JA, Gruebl T, Kirsch J, Muller U, Beyreuther K, Kins S (2007): Beta-amyloid precursor protein can be transported independent of any sorting signal to the axonal and dendritic compartment. J Neurosci Res 85:2580-2590.
Halic M, Blau M, Becker T, Mielke T, Pool MR, Wild K, (2006): Sinning I & Beckmann R. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature 444, 507-511.
Kins S, Lauther N, Szodorai A, Beyreuther K (2006) Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease. Neurodegener Dis 3:218-226.
Kuan YH, Gruebl T, Soba P, Eggert S, Nesic I, Back S, Kirsch J, Beyreuther K, Kins S. PAT1a modulates intracellular transport and processing of amyloid precursor protein (APP), APLP1, and APLP2. J Biol Chem. 2006 Dec 29;281(52):40114-23.
Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Löwer A, Langer A, Merdes G, Paro R, Masters CL, Müller U, Kins S, Beyreuther K. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005 Oct 19;24(20):3624-34. Erratum in: EMBO J. 2006 Feb 8;25(3):653.
Selected publications 2005 and earlier:
Wild K, Sinning I & Cusack S. Crystal structure of an early protein-RNA assembly complex of the signal recognition particle. Science 294, 598-601 (2001).
Weichenrieder O, Wild K, Strub K & Cusack S. Structure and assembly of the Alu domain of the mammalian signal recognition particle. Nature 408, 167-173 (2000).