Nanoscopic Investigation of Living Cellular Complexes with “Conventional” Fluorescent Dyes
Press Release No. 1/2009
6 07 2009
6 07 2009
Heidelberg scientist performs localisation microscopy with green fluorescent protein
Scientists at Heidelberg University have devised an extremely efficient instrument for use in research on cellular processes. It is a combination of the world’s fastest nano light microscope for 3D cell analysis, also developed in Heidelberg, with a new localisation microscopy technique called SPDM (spectral precision distance microscopy). With this new instrument, even several living cells can be studied simultaneously in molecular detail using conventional fluorescent dyes that are well established in biolaboratories, for example green fluorescent protein (GFP). “Up to now, light-optical nanoscopy has been a very intricate business, requiring the use of special photoswitchable fluorescent molecules,” emphasises Prof. Dr. Christoph Cremer of the Kirchhoff Institute of Physics.
Fluorescent dyes have a crucial role to play in high-resolution light microscopy at the nanoscale. To localise adjacent molecules in the “twilight” of the cell and make them independently visible, a temporally convertable light signal is required. So far, signals like this have been supplied by special fluorescent molecules that can be switched on and off by means of light. But, as Prof. Cremer’s latest research has shown, many “conventional” dyes can be switched in this way, as long as certain photophysical conditions are fulfilled. These conditions can be achieved via the so-called “reversible photobleaching” of the fluorescent dyes. According to Professor Cremer there are millions of gene constructs with dyes from the GFP group available in biomedical laboratories all over the world. They could be put into immediate use for this new kind of localisation microscopy.
Fluorescent dyes have a crucial role to play in high-resolution light microscopy at the nanoscale. To localise adjacent molecules in the “twilight” of the cell and make them independently visible, a temporally convertable light signal is required. So far, signals like this have been supplied by special fluorescent molecules that can be switched on and off by means of light. But, as Prof. Cremer’s latest research has shown, many “conventional” dyes can be switched in this way, as long as certain photophysical conditions are fulfilled. These conditions can be achieved via the so-called “reversible photobleaching” of the fluorescent dyes. According to Professor Cremer there are millions of gene constructs with dyes from the GFP group available in biomedical laboratories all over the world. They could be put into immediate use for this new kind of localisation microscopy.
| |
|
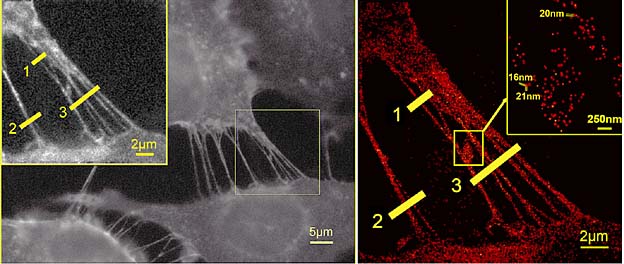
Membrane nanotubes from four breast-cancer cells. Even in the extremely wide field, the molecules labelled by fluorescent dyes from the GFP group can be localised at distances as close as 16 nm.
|
With their SPDM localisation microscopy Christoph Cremer and his team have enhanced the potential of their nanoscope Vertico SMI, which combines far-field light microscopy with extraordinary nanometric accuracy. With the use of visible laser light, this means that not only large cell areas but also cell complexes can be studied two-dimensionally with a spatial resolution of anything down to the range of 10 nanometres. High data acquisition speed makes it possible for the first time to achieve nanoscale 3D recordings of entire (living) cells with a resolution of up to 40 nanometres in real time.
High-density molecule visibility is important in recognising, say, agglomerations of molecules as sites of increased activity. With the nanoscopic technique developed by Professor Cremer, several million individual molecules of a given type can be localised in a wide field of view and recorded within 30 seconds combining up to 2,000 individual images (sufficient for a total picture). This high data-acquisition speed even makes it possible to observe adjacent molecules in nanostructures in living cells. With the extension of SPDM to multicolour co-localisation microscopy, two different protein types can be labelled by conventional fluorescent molecules from, say, the GFP group and detected with different light wavelengths. This, says Professor Cremer, makes it possible to obtain more precise information on potential interactions of individual localised protein molecules in nanostructures than is the case with the conventional FRET method (fluorescence resonance energy transfer).
| |
|
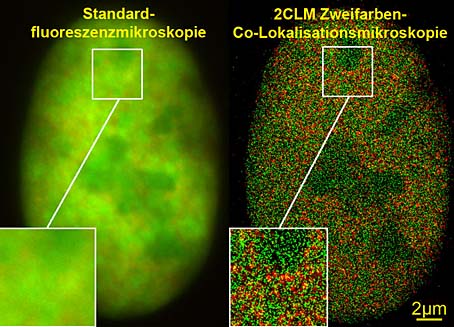
View of the nucleus of a bone-cancer cell. Left: Standard fluorescent microscopes are unable to identify structural details. Right: With 2CLM two-colour co-localisation microscopy many thousands of molecules (120,000) can be clearly distinguished.
|
The combination of the nanoscope with localisation microscopy can be used in pharmaceutical, cell-biological, medical and biophysical research, i.e. wherever molecular imaging is required on a cellular scale. At present, the combination is being employed for cooperative research projects in pharmacology, cardiology, and adult stem-cell research. Further potential uses are studies on the interaction of viruses and cells where the relevant structures are too small for detection by conventional light microscopes, research on age-related neurological degeneration phenomena and cancer research. Determination of the position of individual molecules can furnish new insights on the regulation and activities of genes and proteins or on changes in cellular nanostructures. Other potential uses are in materials research, nano-coating quality control, damage analysis at crack points or the detection of infinitesimal amounts of material in ecological and environmental research.
The Technologie-Lizenz-Büro (TLB) Karlsruhe has been entrusted with the commercialisation of the patent portfolio for nanoscope technology and its applications.
For further information go to www.kip.uni-heidelberg.de/AG_Cremer
Contact
Prof. Dr. Christoph Cremer
Heidelberg University
Kirchhoff Institute of Physics
phone: +49 6221 549252
christoph.cremer@physik.uni-heidelberg.de
General inquiries from journalists should be addressed to
Heidelberg University
Communications and Marketing
Dr. Michael Schwarz
Public Information Officer
michael.schwarz@rektorat.uni-heidelberg.de
Irene Thewalt
phone: +49 6221 542311
presse@rektorat.uni-heidelberg.de
Editor:
Email

