Engineering MHC recognition and biological kinetics of peptides by novel chemistryResearch - Marius Werner |
|
|
|
||
|
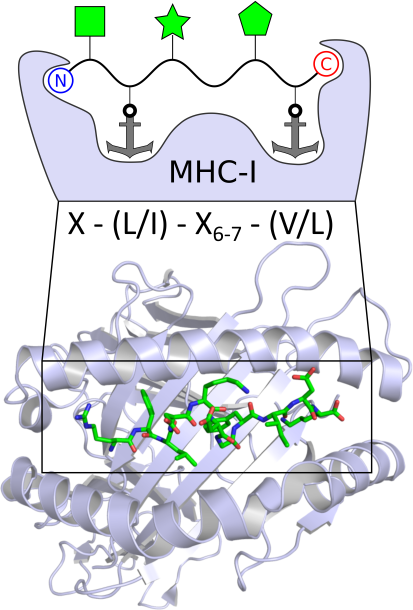
Major Histocompatiblity Complexes (MHC) class I and II are membrane proteins on the cell surface that are important components of adaptive immunity. Simply put, MHC-I and MHC-II can inform T cells about what is going on inside the cell by presenting peptides of processed proteins. Such MHC-peptide complexes bind to T cell receptors and can trigger an immune response. The binding of peptides to MHC-I is controlled by a peptide loading complex in the endoplasmic reticulum, which ensures that peptide epitopes with the highest affinity are bound to MHC-I before being transferred to the cell surface. Peptides in the binding groove of MHC-I are enclosed at the N- and C-terminus, resulting in a size limitation of 8-10 residues. MHC-II has an open binding groove and typically accepts peptides with 13-25 residues. In both cases, the peptides bind to the MHC molecules in specific pockets with well-matched anchor residues. The goal of my research is to develop peptides with high binding affinity to MHC-I and MHC-II for use as novel peptide vaccines or in cancer immunotherapy. Peptides are synthesized by solid-phase peptide synthesis (SPPS) and can be modified by late-stage functionalization. Promising candidates are evaluated by in vitro assays. |

