Current Research
In recent years, significant progress has been made to understand the generation of neuronal diversity. One of the key models used for the study of this question are the peripheral sympathetic ganglia of mammals and birds. They contain two neuron populations which develop from the same precursor pool but differ in their transmitter, noradrenaline or acetylcholine ,respectively. Analyzing the expression of genes coding for the transmitter-synthesizing enzymes, our interest is to identify growth factors and transcription factors which are crucially involved in the segregation of neuronal fates. In addition, we analyze signals regulating the expression of general neuronal genes such as those coding for synaptic proteins to understand how general neuronal differentiation and population-specific development are coordinated.
Noradrenergic induction – specifying neurons by coordinate gene expression
Noradrenergic properties are induced early during sympathetic neurogenesis by BMP growth factors and Phox2transcription factors in birds and mammals. Comparing the expression of tyrosine hydroxylase and dopamine ß- hydroxylase, we could show that these two enzymes of the noradrenaline biosynthesis cascade are expressed at the same time during sympathetic neuron differentiation. The induction by the same growth and transcription factors suggests regulation of these enzymes as a synexpression group that is conserved in evolution from birds to mammals.
Genes coding for synaptic proteins – induction of general as compared to population-specific properties
Synaptic proteins are more generally expressed in neurons than individual transmitter-synthesizing enzymes. They function in different aspects of transmitter release and synapse organization. Over expression studies show that synaptotagmin I and neurexin I can be induced by BMP growth factors and Phox2 transcription factors in sympathetic precursors. Thus, it appears that induction of general and population-specific neuronal characters involves common mechanisms. In the closely related adrenal chromaffin cells, different regulation of these genes provides evidence forearly segregation of neuronal and endocrine lineages.
Generation of neuronal diversity in sympathetic ganglia
Choline acetyltransferase (ChAT) and the vesicular racetylcholine transporter (VAChT) are two main features of cholinergic neurons which are coordinately transcribed from the cholinergic gene locus. The loss of ChAT and VAChT expression in sympathetic ganglia of c-ret mutant mice demonstrates that signaling via receptors for growth factors of the GDNF family is necessary for the development of cholinergic sympathetic neurons. Expression of c-retin cholinergic sympathetic neurons of the chick embryo suggests that its role may be evolutionary conserved. Whereas c-ret acts late during maturation in sympathetic ganglia, factors involved in the early induction of cholinergic properties remain to be determined.
Future Research and Goals
Our interest is to understand how signalling by growth factors and regulation by transcription factors coordinates the acquisition of general neuronal features and the diversification of neuronal lineages. Analyzing developmental expression patterns of genes coding for different synaptic proteins which are expressed across many neuron populations, we ask whether different neuron classes share common differentiation pathways. We then search for the transcriptional regulators involved to understand whether general neuronal features are induced by population specific or rather by widely expressed transcription factors. In comparison, we investigate the expression of genes coding for enzymes of neurotransmitter biosynthesis and transport which are specific for defined neuron populations. Understanding the factors involved in their expression, we hope to comprehend how general and population-specific aspects of neuronal differentiation are coordinated.
Selected Publications
Huber, K., and Ernsberger, U. (2006) Gene Expr. 13, 133-139
Ernsberger, U., Rohrer, H. (2006) In “Transcription factors in the nervous system”,
Ernsberger, U. et al. (2005). Cell Tissue Res 319, 1-13
Burau, K., et al. (2004). Eur J. Neurosci. 20, 353-362.
Ernsberger, U. (2004).. In “Molecular Biology of the Neuron”, 2nd ed.
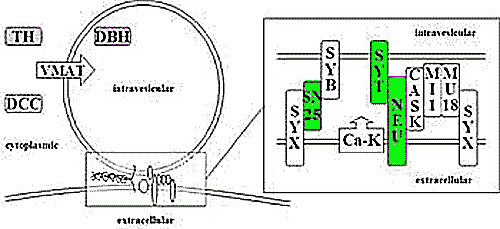 |
| Fig. 1: Schematic representation of a transmitter vesicle, the transmitter synthesizing enzymes tyrosine hydroxylase (TH) and dopamine ß-hydroxylase (DBH), and certain proteins involved in vesicle fusion and transmitter release. SNAP-25 (S25), synaptotagmins (SYT), neurexins (NEU), and voltage-gated calcium channels (Ca-K) are involved in different functional aspects of transmitter release and are addressed in our studies. |
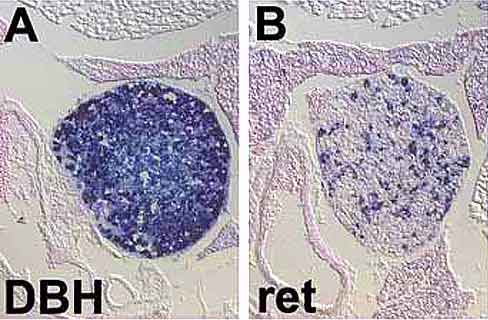 |
| Fig. 2: Detecion of mRNAs for dopamine ß-hydroxylase (DBH) (A) and ret (B) by in situ hybridization shows that the vast majority of neurons in the superior cervical ganglion are noradrenergic. A subpopulation of neurons expresses ret. |
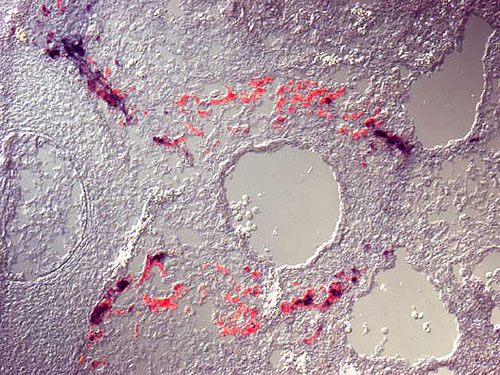 |
| Fig. 3: Expression of mRNAs for tyrosine hydroxylase (TH) [red] and neurofilament M (NF-M) [black] in sympathoadrenal precursors. Distinct expression levels for the subclass-specific marker TH and the general neuronal marker NF-M demonstrates different regulation of gene expression in neuronal and neuroendocrine cells. |

