The Search for a Molecular Mirror Image
28 November 2013
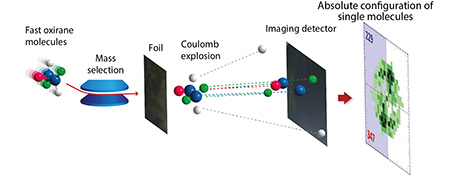
Image source: P. Herwig, K. Zawatzky, O. Trapp, H. Kreckel
In a complex experiment, Heidelberg researchers have succeeded for the first time in determining the absolute configuration of a chiral compound by means of direct imaging. They were able to conclude unequivocally whether they were looking at images or mirror images of the investigated molecules – something that is not possible with current methods without any risk of errors. The interdisciplinary project involved scientists of Heidelberg University’s Institute of Organic Chemistry and the Max Planck Institute for Nuclear Physics in Heidelberg, with the cooperation of experts of the Weizmann Institute of Science in Rehovot (Israel). The research findings will be published in “Science”.
Many molecules exist as image and mirror image, much like our left and right hands. This property of the molecules – known as “chirality”, hence the term “chiral” molecules – may result in considerable differences in terms of the molecules’ effect and properties. This concerns numerous pharmaceuticals, and also odorants like carvone, an essential oil whose mirror images smell like caraway seed or mint. The fact that molecules can occur as image and mirror image was discovered in 1847 by the French chemist and microbiologist Louis Pasteur. Prof. Dr. Oliver Trapp of the Institute of Organic Chemistry at Heidelberg University calls this discovery the birth of stereochemistry, the scientific discipline that examines the spatial structure of molecules.
The Heidelberg scientist explains that it was not until 1951 that anomalous x-ray diffraction first offered absolute proof of whether a given image was in fact the image or rather the mirror image of a molecule. “However, to determine the absolute configuration with this method we must make some theoretical assumptions that may still lead to errors and cannot always provide definite results”, says the chemist. “Until now, there was no independent method for a direct experimental imaging of a molecule’s chirality, especially in the gas phase.”
In preparation of the experiment, Prof. Trapp’s team first designed a suitable chiral compound and then synthesised it with a defined chirality in a sophisticated process. The molecules of this isotope-labelled compound were then ionised in close cooperation with the team of Dr. Holger Kreckel and Prof. Dr. Andreas Wolf at the Max Planck Institute for Nuclear Physics. In the ionisation process, one electron of the respective molecule is removed, turning the molecule into a positively charged ion. The molecule ions were then accelerated to extremely high speeds in a particle accelerator. To obtain a direct image of the molecules, the Heidelberg researchers used a technique developed by Prof. Dr. Zeev Vager at the Weizmann Institute, which they “refined to a considerable extent”, as Prof. Trapp explains.
The scientists used a mechanism called a Coulomb explosion, which allows the accelerated molecule ions to penetrate a very thin diamond foil in less than a femtosecond. This process causes a “molecular striptease” in which the bonding electrons are stripped away and the atoms, which now have a strong positive charge, repel each other. The relative arrangement of the atoms, however, is preserved, leading to the formation over time of a larger three-dimensional image of the molecule that retains its basic geometry. “We chose the experiment so that the molecule structure grew to a few centimetres and could be clearly identified”, say the researchers.
To perform the measurements required for the imaging process, the scientists had to optimise the detector system to enable the simultaneous spatial and time-resolved detection of up to five atoms. “That was the prerequisite that allowed us to directly image the absolute configuration of a molecule with a defined chirality in an experiment”, emphasises Prof. Trapp. “In future, the imaging method involving the Coulomb explosion in combination with high-resolution mass spectrometry will allow us to look even at molecule fragments and determine whether they are images or mirror images. From there, it is only another step to the molecular sequencing of the absolute configurations of several stereocentres in complex molecules such as biomolecules.”
The organic chemists and nuclear physicists are already planning their next pioneering experiments. The chiral molecules could, for instance, be accumulated in an ion storage ring prior to the Coulomb explosion, which would open up completely new perspectives for research, as the Heidelberg scientist states.
Original publication:
P. Herwig, K. Zawatzky, M. Grieser, O. Heber, B. Jordon-Thaden, C. Krantz, O. Novotný,
R. Repnow, V. Schurig, D. Schwalm, Z. Vager, A. Wolf, O. Trapp and H. Kreckel: Imaging the absolute configuration of a chiral epoxide in the gas phase, Science (29 November 2013)

