Zika Virus: Structure of Key Enzyme Uncovered
8. Juli 2016
Heidelberg and Lübeck research collaboration
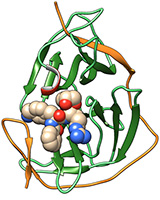
Abb: Hilgenfeld et al.
Using X-ray structure analysis and a highly potent enzyme inhibitor, researchers from the universities of Heidelberg and Lübeck succeeded in clarifying the structure of a key enzyme of the Zika virus at the atomic level. Heidelberg researchers Dr Christoph Nitsche and Prof. Dr Christian Klein designed and synthesised the inhibitor. Their work is part of a project funded by the German Research Foundation being conducted at the Institute of Pharmacy and Molecular Biotechnology (IPMB) of Ruperto Carola. The research is focussed on producing substances that effectively and selectively inhibit the protease of flaviviruses – such as Zika, dengue and West Nile virus.
What makes the substances produced in Heidelberg special is their ability to bind very tightly to their macromolecular target, the protease of the virus, which is an enzyme essential for replication. According to Prof. Klein, a tight interaction is especially desirable for effectively deactivating the points of attack of infectious agents. The substances also help to shed light on the three-dimensional structure of these specifically created molecule complexes, which in the case of Zika consist of the enzyme inhibitor and the protease of the virus. The Lübeck researchers based their studies on this inhibitor-protease complex. To uncover the atomic-level structure of the protease of one of the key enzymes of the Zika virus, the investigators at the Institute of Biochemistry at the University of Lübeck used X-ray structure analysis, which identifies the position of the atoms in three dimensions.
The researchers hope the results of their research, which were published in the journal "Science", will spur the development of new forms of therapy. Prof. Klein emphasises that further comprehensive studies are needed to develop potential medications. "Using our enzyme inhibitor and the structure decoded by our colleagues in Lübeck, however, we have laid the basis for a targeted search for drug candidates."

