Current Research
1) Gangliosides in the Pathogenesis of Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common chronic neurodegenerative disorder of the CNS and the leading cause of dementia. AD is characterized clinically by a progressive loss of memory and other cognitive functions. Accumulating research evidence indicates that neuroinflammation, obesity, and type-2 diabetes significantly increase the risk of developing the sporadic form of AD.
Neurotoxic amyloid-β (Aβ) species are generated at plasma cell membranes through amyloidogenic cleavage of the amyloid precursor peptide (APP) by sequential activity of β- and γ-secretases. During the past decade, it has been hypothesized that oligomeric Aβ species mainly exert the neurotoxic properties in AD. Furthermore, oligomeric Aβ species have been shown to induce a loss of insulin receptors from the neuronal cell surface, which contributes to neuronal insulin resistance and subsequent neurodegeneration.
We aim to clarify the role of gangliosides in the etiology and progression of AD. Gangliosides are a specific class of membrane lipids with a hydrophobic ceramide membrane anchor and a hydrophilic carbohydrate chain typically containing one or more sialic acid residues. Glucosylceramide synthase (GCS) is the key enzyme in ganglioside biosynthesis (Fig. 1A).
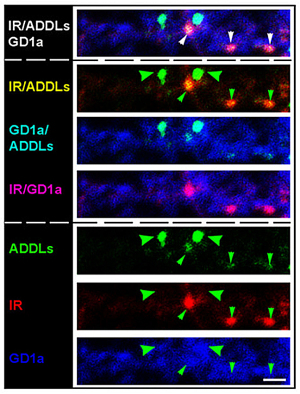
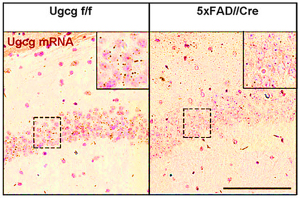
We have detected that ADDLs form complexes with dendritic insulin receptors and ganglioside GD1a (Fig. 1B). Ganglioside biosynthesis in cultured neurons can be efficiently inhibited by treatment with the specific GCS inhibitor GENZ-123446 (GENZ, Fig. 1C). Strikingly, GENZ treatment increases the viability of cultured neurons exposed to ADDLs (Fig. 1D) and counteracts the ADDL-induced loss of surface insulin receptors (Fig. 1E). Consequently, GENZ-treated neurons maintain their insulin sensitivity upon exposure to ADDLs (Fig. 1F). We could furthermore show that genetic GCS deletion in forebrain neurons, inter alia the hippocampus (Fig. 1G) and cerebral cortex, protects AD mice from neurodegeneration (Fig. 1H).
In summary, we propose that GCS inhibition increases neuronal viability in models of AD. GCS inhibition furthermore stabilizes the levels of functional insulin receptors on the neuronal surface. We propose that gangliosides facilitate ADDL-mediated insulin receptor internalization, occurring in caveolin-1-containing caveolae (Fig. 2). Thus, we suggest that gangliosides may constitute a novel therapeutic target against AD.
Figures 1A-H. Inhibition of ganglioside biosynthesis protects neurons in models of Alzheimer’s disease.
Figures 1B-H modified from Herzer et al. (2016) Acta Neuropathol Commun 4:103.
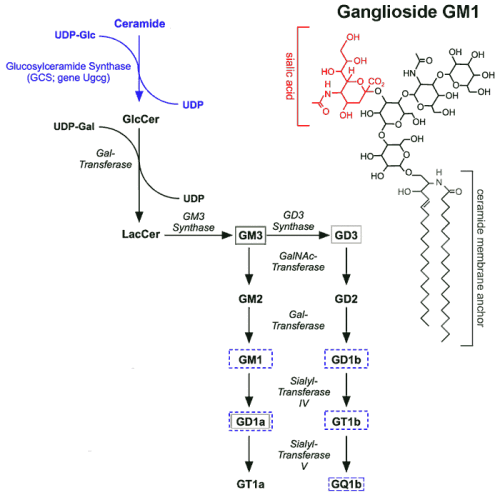 |
| A: Ganglioside biosynthesis starts with the formation of glucosylceramide by the key enzyme glucosylceramide synthase (GCS, gene Ugcg). Different ganglioside species are synthesized by sequential addition of carbohydrate moieties to the precursor lactosylceramide. |
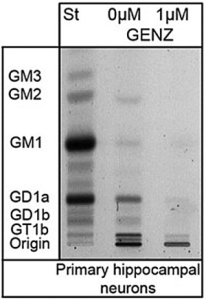 |
| C: Treatment with the GCS inhibitor GENZ-123446 inhibits ganglioside biosynthesis in neurons, as shown by thin layer chromatography. |
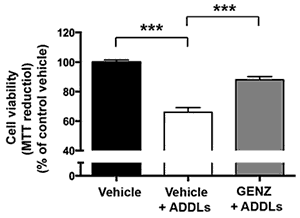 |
| D: GENZ treatment protects mHippoE-14 cells from ADDL toxicity. |
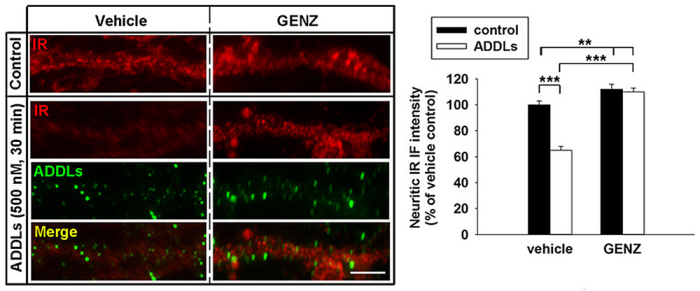 |
| E: ADDL-mediated loss of insulin receptors from the dendritic surface can be prevented by GENZ treatment of primary neurons. |
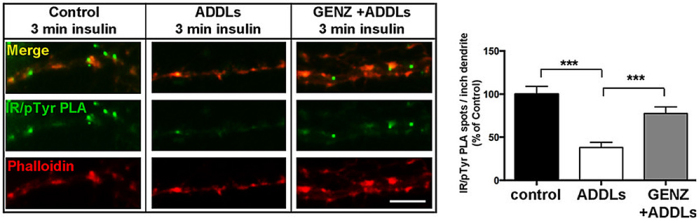 |
| F: Proximity ligation showing dendritic phosphorylated insulin receptors. GCS inhibition by GENZ restores insulin sensitivity in neurons exposed to ADDLs. |
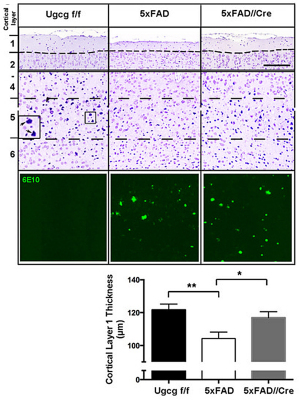 |
| H: Cresyl violet staining of cortical layers of 7 months old 5xFAD//Cre mice shows that cortical layer 1 thickness, reflecting neuronal density in layer 5, is reduced in 5xFAD mice, but maintained in 5xFAD//Cre mice. This indicates that neurons in 5xFAD//Cre mice are protected from neurodegeneration even though Aβ plaques are present (Aβ deposits in cortical layer 5 are stained by the antibody 6E10). |
|
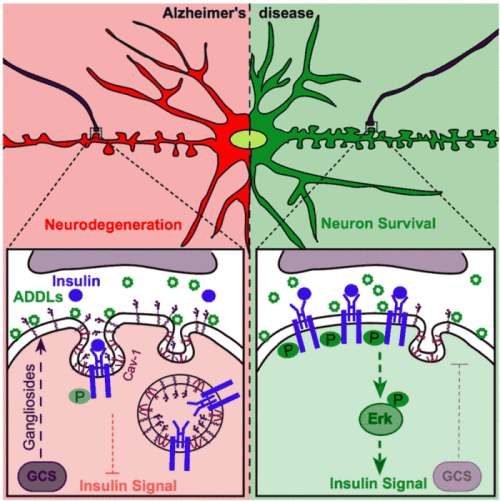 |
| Figure 2. GCS inhibition increases neuronal viability and counteracts the ADDL-induced removal of insulin receptors from the neuronal surface. We propose that gangliosides facilitate ADDL-induced internalization of the insulin receptors via caveolin-1-containing caveolae. GCS inhibition can prevent the loss of surface insulin receptors and maintain neuronal insulin sensitivity in models of Alzheimer’s disease. Figure from Herzer et al. 2016. Acta Neuropathol Commun 4:103. |
We are currently investigating how gangliosides in neurons, astrocytes, and microglia contribute to AD pathology.
2) Gangliosides in the Regulation of Body Weight and Energy Homeostasis
The prevalence rates of obesity and associated co-morbidities, such as cardiovascular diseases and type-2 diabetes, are continuously rising worldwide. Therefore, major research efforts have been put into understanding the underlying molecular mechanisms, in order to develop prevention or treatment strategies.
Energy homeostasis and body weight are regulated by CNS neurons chiefly located in the hypothalamus. Hypothalamic neuronal subpopulations sensing energy signals, such as leptin or insulin, adapt neuronal firing and neuropeptide expression in order to adjust the energy expenditure of the body to energy intake. A malfunction of these fine-tuned systems results in metabolic deregulation, which subsequently leads to body weight divergence.
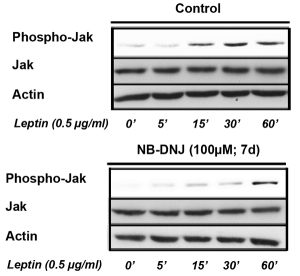
Recently, we have unraveled a novel mechanism as to how hypothalamic neurons regulate body energy homeostasis. Leptin and insulin receptor function in hypothalamic neurons critically depends on an adequately balanced expression of gangliosides. We show that gangliosides in hypothalamic neurons regulate leptin receptor signaling (Fig. 3A). With the help of the innovative proximity ligation assay (PLA, Duolink®), we could detect close molecular interaction dynamics between hypothalamic neuronal leptin receptors and gangliosides GD1a and GM1 upon leptin stimulation (Fig. 3B). We have found out that a fine-tuned balance of neuronal GCS expression in the hypothalamus is necessary for the control of body weight and adipose tissue homeostasis (Fig. 3C).
Figure 3A-C. Leptin receptor function in hypothalamic neurons depends on interactions with gangliosides in the neuronal cell membrane.
Figures modified from Nordström et al. (2013) PLoS Biol 11(3).
| A: Leptin receptor signal transduction in hypothalamic neurons requires adequate expression of gangliosides. Hypothalamic neurons treated with the GCS inhibitor NB-DNJ display decreased leptin sensitivity. |
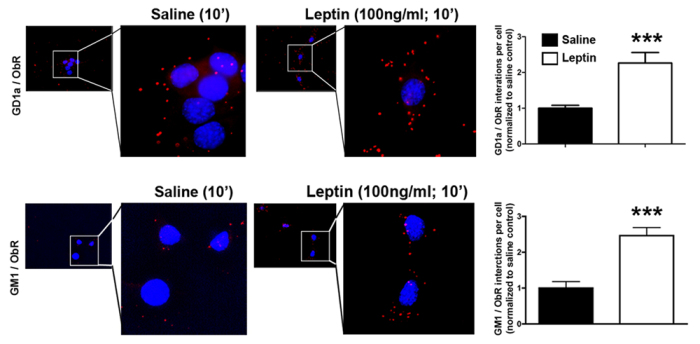 |
| B: Gangliosides closely interact with leptin receptors, as shown by proximity ligation (PLA). |
| 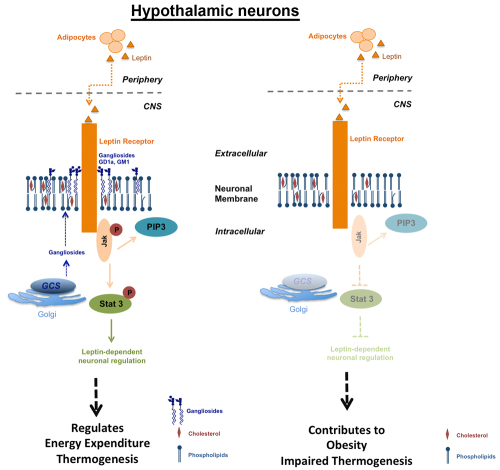 |
| C: Proposed model of ganglioside regulation of leptin receptors and subsequent body weight. |
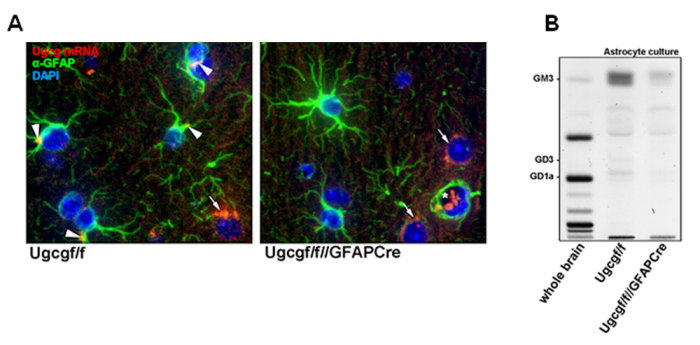
3) The Role of Astrocytic Gangliosides in High Fat Diet (HFD)-induced Hypothalamic Inflammation (Project Leader: Dr. Silke Herzer)
Obesity and its associated co-morbidities has become one of the biggest health risks of industrialized affluent societies. In most obese individuals, hypothalamic regulation of energy balance is impaired. Even though current research has provided comprehensive data on the function of hypothalamic neuronal networks, the mechanisms underlying neuronal alterations in obesity are not yet completely understood.
Inflammation in adipose tissue and the liver of obese individuals has been reported frequently. However, during the recent years experiments could provide evidence for inflammatory processes in the hypothalamus of HFD-fed rodents long before the onset of obesity. Activation of astrocytes and microglia has been documented as a characteristic feature in the initiation of hypothalamic inflammation. It is assumed that long-term consumption of HFD can lead to sustained glial activation and chronic inflammation. This initiates a vicious circle of impaired energy balance and hypothalamic inflammation, eventually perpetuating progressive weight gain and obesity. However, molecular mechanisms as to how HFD-derived metabolic factors such as saturated fatty acids can initiate a hypothalamic inflammatory response are poorly understood.
Therefore, we aim to unravel the function of gangliosides in hypothalamic astrocytes. We are currently studying whether astrocyte-specific inhibition of ganglioside biosynthesis in mice can alter HFD-induced astrocytic activation and subsequent hypothalamic inflammation and obesity. These findings are expected to shed more light on the role of gangliosides in the HFD-induced activation process of astrocytes and the consequent hypothalamic inflammation.