Current Research
It is now commonplace that principles guiding embryonic development in humans and in animals are very similar on a molecular level and that genes involved in development also play a role in human disease. The Division of Molecular Embryology is studying mechanisms regulating embryonic cell differentiation in Xenopus and mouse. Our aim is to characterize molecular mechanisms relevant for the formation of the embryonic body axis. To this end, we identify developmental control genes, investigate what their biological role and biochemical mode of action is and how they are regulated. We specifically address the following two main topics: I. Molecular mechanisms of Wnt/β-catenin signalling during early vertebrate development. Elucidation of the functions of of Dickkopf (Dkk) proteins, a class of Wnt inhibitors, is a major focus. II) Molecular mechanis of embryonic reprogramming, with the aim to elucidate molecular mechanisms regulating embryonic pluripotency.
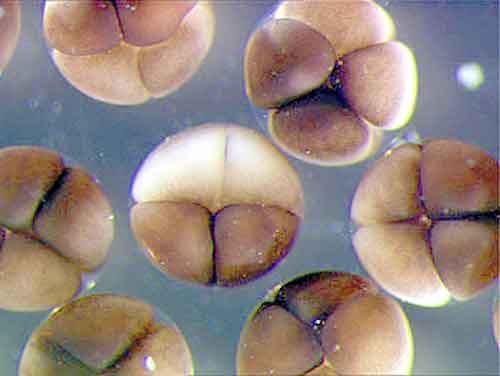 |
| Fig. 1: Xenopus embryos at 4-cell stage |
Mechanisms of Wnt signaling in early vertebrate development The Wnt/β-catenin pathway plays an eminent role in development and disease. We showed that in Xenopus embryogenesis, Wnt/β-catenin signaling plays an important role in antero-posterior patterning of the early central nervous system. Our results demonstrated a posteriorizing gradient of Wnt signalling, which regulates dose-dependently positional information from the forebrain to spinal cord. Importantly, we revealed an endogenous antero-posterior gradient of Wnt/β-catenin signalling in the presumptive neural plate of the Xenopus gastrula. Together with other data this led to the proposal of perpendicular activity gradients of Wnt and BMPs, which regulate early CNS patterning (Fig. 2).
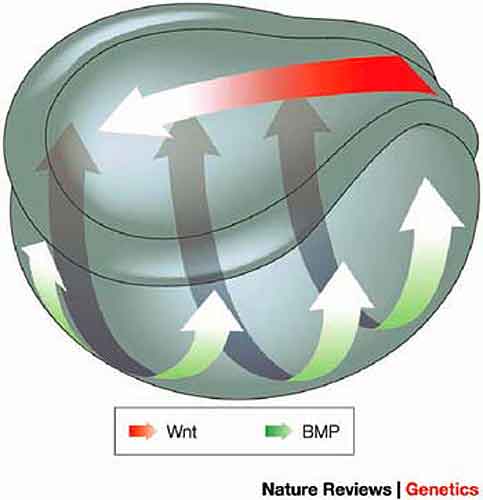 |
| Fig. 2: Double-gradient model for A-P and D-V axial patterning. The model shows how perpendicular activity gradients of Wnts and bone morphogenetic proteins (BMPs) regulate head-to-tail (A-P) and dorsal-ventral (D-V) CNS patterning. The colour scales of the arrows indicate the signalling gradients; arrows indicate the spreading of the signals. Patterning begins at gastrula stages, but for clarity, it is depicted in an early amphibian neurula. The formation of head, trunk and tail requires increasing Wnt activity. Anterior and posterior are to the left and right respectively. |
The model shows how perpendicular activity gradients of Wnts and bone morphogenetic proteins (BMPs) regulate head-to-tail (A-P) and dorsal–ventral (D-V) CNS patterning. The colour scales of the arrows indicate the signalling gradients; arrows indicate the spreading of the signals. Patterning begins at gastrula stages, but for clarity, it is depicted in an early amphibian neurula. The formation of head, trunk and tail requires increasing Wnt activity. Anterior and posterior are to the left and right, respectively. We previously identified an important regulator of Spemann’s organizer, dickkopf1(dkk1), member of a new family of secreted proteins. Dkk1 encodes a Wnt inhibitor and it is functions in embryonic head development (Fig. 3). While the gene was initially characterized as a developmental regulator, dkk1 is gaining increasingly medical attention, since it has been implicated in bone physiology and myeloma by others. We currently study the role of Dickkopf genes and their receptors Kremen and LRP6 during Xenopus and mouse development, including CNS. Towards this end we identify interacting proteins of these proteins and elucidate their role in Wnt signalling and early development. This led to the recent discovery of Rspondins and Casein kinase 1 gamma as novel Wnt regulators, which are also involved in CNS patterning.
Reprogramming
Another line of research in our laboratory is devoted to the question of reprogramming i.e. the artificial process of transformation of a somatic into a pluripotent cell. The interest is two-fold. First, by identifying regulatory genes that control the state of pluripotency we hope to learn how the differentiated vs. pluripotent state is regulated. Second, this knowledge may be used to generate human isogenic pluripotent stem cells for regenerative medicine. Towards this end we have developed partial embryonic reprogramming of entire somatic cells by using Xenopus egg extracts. Screening for factors required for reprogramming identified the chromatin remodelling ATPase BRG1.
Furthermore, we have identified a molecular mechanism that is involved in demethylation and re-activation of pluripotency genes, such as oct4. This mechanism involves the stress response gene Gadd45, which acts by recruiting DNA repair to sites of demethylation. Methlyated cytosines are excised and replaced by unmethylated nucleotide, thereby leading to demethylation. This is a novel epidgenetic mechanism of gene activation. We currently study the role of this process during early Xenopus development and we screen for factors that mediate the site specific targeting of Gadd45 to sites of demethylation in the genome.
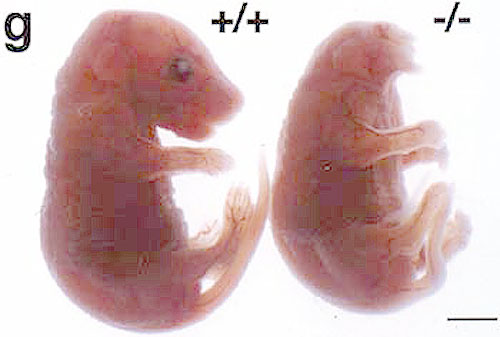 |
| Fig. 3: Day 17 mouse embryos wild type (left) and dkk1 null mutant. |

