Current Research
Cross talk between excitatory and inhibitory synapses
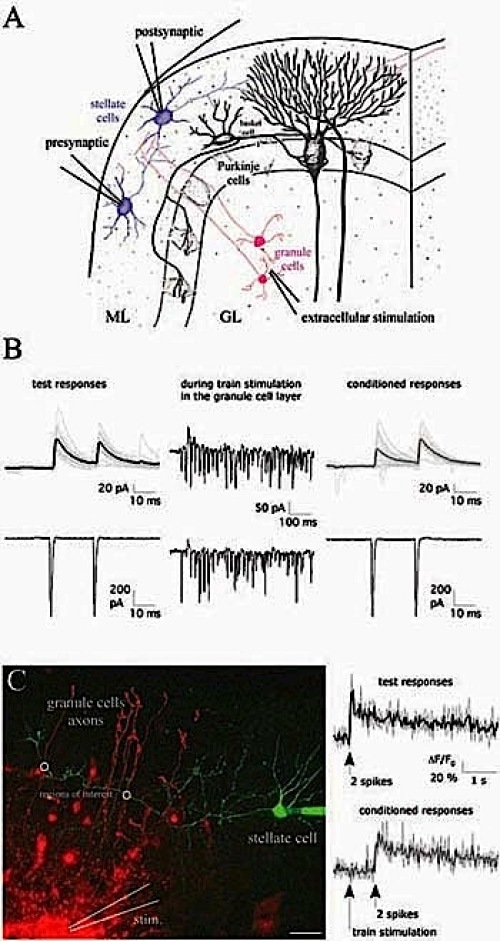
GABA release from cerebellar molecular layer interneurons can be modulated by presynaptic glutamate and/or presynaptic GABAB receptors when perfusing the respective agonists. However, it is unclear how release and potential spillover of endogenous transmitter lead to activation of presynaptic receptors. High frequency firing of granule cells, as observed in vivo upon sensory stimulation, could lead to glutamate and/or GABA spillover. Using paired recordings from connected stellate cells and imaging techniques (Fig. 1), we study how sustained glutamatergic activity in the granule cell layer modulates GABA release in the molecular layer.
 |
|
Fig. 1: (A) Scheme of a cerebellar section including electrodes for paired recordings (ML, molecular layer; GL, granule cell layer). (B) Test and conditioned IPSCs in the postsynaptic cell (-35 mV; upper row) were evoked by paired depolarization pulses elicited in the presynaptic cell (-60 mV; lower row). Conditioned eIPSCs were preceded by train stimulation (30 stimuli at 50 Hz). (C) A stellate cell was filled with 100 μm Oregon Green BAPTA-1, and granule cells were electroporated (500 μm Alexa Fluor 594) during train stimulation. Axonal Ca 2+ transients were evoked by paired-pulse spiking (20ms) and were acquired by two-photon microscopy. Scale bar= 20 μm. |
Hippocampal synaptic plasticity
Long-term potentiation (LTP) or long-term depression (LTD) of synaptic transmission are widely acknowledged as the primary candidate manifestation of the cellular basis of learning and memory. Most forms of LTP and LTD follow a Hebbian rule, requiring coincident presynaptic and postsynaptic activity during their induction. The presynaptic signal is thought to be glutamate release, whereas the postsynaptic signal is depolarization, allowing influx of calcium through NMDA receptors and/or voltage-gated calcium channels, and further supported by release of calcium from intracellular stores. For the expression of synaptic plasticity, presynaptic and/or postsynaptic mechanisms can be involved. Within the hippocampus, mossy fiber synapses onto CA3 pyramidal neurons have unique features distinct from other excitatory synapses. Regarding synaptic plasticity, the most striking difference is that NMDA receptor-independent LTP can be induced upon high frequency presynaptic stimulation. Given that mossy fibers activate substantial NMDA receptormediated currents in CA3 neurons, the role of the NMDA receptors at this synapse is unclear. We investigate whether patterns of activity different from high frequency stimlulation can recruit NMDA receptors to induce synaptic plasticity at mossy fiber-to-CA3 synapses in acute slices of P21 rodents. Fig. 2 illustrates one example of spike timing-dependent plasticity (STDP; +10 ms), which induces LTP.
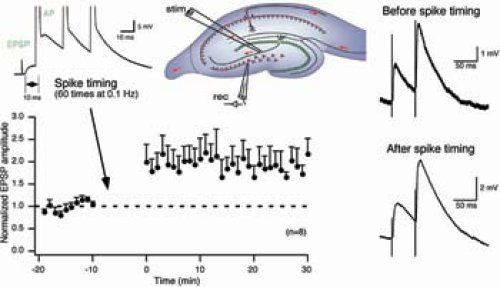 |
| Fig. 2: Excitatory postsynaptic potentials (EPSPs) were evoked by mossy fiber stimulation (see scheme) in CA3 neurons of P21 rats in 10 μm bisculline and 10 μm glycine. LTP was induced by 60 EPSP-action potential (AP) pairs. The three APs were evoked at 50 Hz and delayed by 10 ms after the evoked EPSP. The APs of the voltage traces are clipped. Note that the paired-pulse facilitation did not change after spike timing. |
NMDA receptor subtypes: functional properties and localization
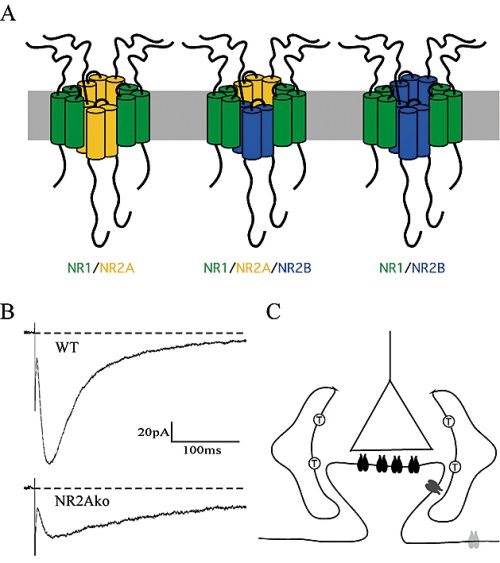
NMDA receptors are heterotetrameric complexes composed of two NR1 and two NR2 subunits (NR2A-D) subunits. Increasing expression of NR2A in hippocampal neurons after birth leads to the formation of diheteromeric NR1/NR2A and NR1/NR2B as well as triheteromeric NR1/NR2A/NR2B receptors (Fig. 3A). Despite compelling evidence for triheteromeric NMDA receptors, the functional role of these receptors in synapses is still unknown. We are currently investigating deactivation (Fig. 3B) and peak open probability of synaptic NMDA receptor subtypes in acute hippocampal slices of wild-type and NR2A knockout mice as these functional properties are very different between recombinant NR1/NR2A and NR1/ NR2B receptors.
 |
|
Fig. 3: (A) Expression of two different NR2 subunits within a neuron can lead to the formation of three distinct NMDAR subtypes. (B) NMDA receptor-mediated EPSCs were evoked in CA1 neurons of P28 wild-type (WT) and NR2A knockout (NR2Ako) mice in 10 μm bicuculline, 5 μm NBQX and 10 μm glycine at -40mV. (C) Schematic drawing of an excitatory synapse containing a presynaptic terminal (triangle) and a postsynaptic spine carrying synaptic NMDA receptors (black paired structures). Two astrocytes ensheath the synapse and express glutamate transporters (T) whose activity influences the activation of perisynaptic NMDARs (dark gray). An extrasynaptic NMDAR is present in the dendritic shaft (light gray). |
Besides subunit composition, distinct NMDA receptor functions likely also depend on the spatial distribution of NMDA receptor subtypes within a neuron. Certain interactions of NMDA receptor subunits with other transmembrane receptors (e.g., Dopamine) and/ or intracellular signaling molecules (e.g., kinases or phosphatases) may occur at synaptic but not at peri- or extrasynaptic sites (Fig. 3C). Importantly, these interactions change during postnatal development when scaffolding and signaling complexes reorganize in the postsynaptic densities coinciding with increasing expression of a second NR2 subunit. We investigate distinct signaling pathways in apical and basal dendrites of CA1 neurons by means of protein overexpression or protein knockdown using short interfering RNAs (siRNAs).

