Current Research
Cnidarians and the origine of lower MetazoamsThe origin of the nervous system and the evolution of multi-cellular animals (Metazoa) from single cell organisms is largely unclear at present. The first animals that developed a nervous system are the cnidarians (coralls, sea anemoones and jelly fish). These ancient animals (>500 million years ago) have a simple nerve net and they are important to understand the origin of a central nervous system (CNS). To trace back the evolution of the nervous system, we are analysing developmental processes and neurogenesis in the freshwater polyp Hydra and the sea anemone Nematostella Genome projects in Hydra and Nematostella have now revealed the existence of an extensive set of conserved gene families and developmental pathways regulating embryogenesis throughout higher metazoans like worms, flies and vertebrates. Wnt and BMP signallingThe molecular nature of signalling centers (organizers) plays a pivotal role in the origin and evolution of metazoan body axes. Our lab has cloned wnt/wg genes from Hydra and Nematostella (Fig.1). The families of Wnt/Wingless secreted proteins act as short-range inducers and long-range organizers in axis formation, organogenesis and tumour formation in vertebrates. In cnidarians, the canonical Wnt signalling pathway is expressed in the polyp’s oral signalling center (organizer), which corresponds to the blastopore of metazoan embryos. In Nematostella different wnt genes form staggered expression domains along the oral-aboral axis (“wnt-code”), governing axial differentiation, and neuronal differentiation of early multi-cellular animals. Notably, an orthologue to vertebrate dickkopf-1, -2, and -4 genes is expressed in the body column, complementary to the expression domain of the wnt/β-catenin/tcf/brachyury genes. Dickkopf proteins, which are major Wnt antagonist in vertebrates, also act in cnidarians and we propose that they also promote neuronal differentiation. We are currently testing these hypotheses by using transgenic lines and promotor constructs for wnt, dkk, and bmp genes as well as by testing corresponding recombinant Hydra and Nematostella proteins. |
||
|
||
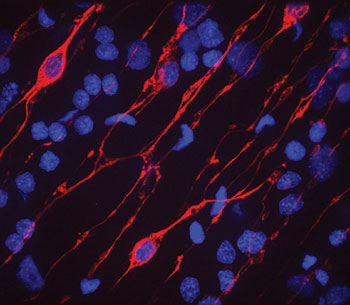
Wnt signalling and cell adhesionTo analyse the potential link between cell signalling and cell adhesion during organizer formation we have identified a Hydra Cadherin (HyCad) by its physical interaction with Hydra β-Catenin in a yeast two-hybrid screen. We propose that a basic function of the Cadherin-Catenin complex was to stabilize an autocatalytic feedback loop leading to the definition of signalling centers. We test this hypothesis by ectopic expression and promoter analysis of both genes. The evolutionary origin of the central nervous systemThe cnidarian nerve net is diffuse and no brain-like structures have been reported. The goal of our work is to uncover the molecular mechanism of neurogenesis in cnidarians and to understand how it is related to the origin, evolution and patterning of neurogenesis among the metazoans (Fig. 2) |
||
|
||
|
Comparisons between vertebrates and insects have revealed a great deal of conservation also in the genetic control of neurogenesis. This suggests that the common ancestor of Bilateria used the same set of genes to regulate neurogenesis. Yet, not all genes seem to be conserved between insects and vertebrates. Dkk proteins for example, which are important antagonists in the Wnt pathway and play a role in suppressing the anti-neuronal function of wnt genes, were not identified in insects and nematodes. Furthermore, zic genes, which have a conserved role in neurogenesis in all vertebrates downstream of Chordin and upstream of bHLH transcription factors, have not been reported to act in neurogenesis of insects. We are therefore interested to learn how these regulatory genes are activated by the cnidarian patterning system. Imaging the dynamics of cnidarian neuronal differentiationWe use stable transgenes of Hydra interstitial cells to analyze neuronal differentiation in embryos, intact polyps, and aggregates. Neuronal cells arise from pluripotent interstitial stem cells that differentiate into several populations of neuronal cells (ganglion cells, sensory neurons and nematocytes). Commitment of these cells occurs in the body column and precursor cells can migrate to the sites of final differentiation. This stem cell system is highly reminiscent to the neural crest system in vertebrates. We analyze the behaviour of proneuronal cells along the oral aboral axis in vivo and under various conditions by using confocal laser scanning microscopy (CLSM), spinning disc confocal microscopy, and 2-photon microscopy. We also analyze the mechanisms of recruitment of proneuronal cells in Nematostella. This is particularly exciting since previous work has shown that neuronal cells arise in more basal cnidarians directly from precursor cells located in the ectodermal epithelium. Important questions answered by this set of experiments are, whether precursor cells arise by asymmetric cell division, whether they undergo additional proliferation steps, and how the pattern of neuronal and nematocyte recruitment is related to thee axial patterning of the polyp. Proteom of nematocytesThe most conspicuous character of Cnidarians is the presence of nematocysts, which are complex exocytotic organelles formed inside a specialized neuronal cell type, the nematocyte (stinging cell). Nematocytes are used for catching prey, and their discharge is one of the fastest events in biology (we showed that the initial phase of discharge is 700 nanoseconds short generating an acceleration of 6,000,000 g). The forces sustained during explosive discharge require extraordinary mechanical strength and elastic modulus in the capsular wall material. Although we made substantial progress in unravelling the molecular structure of nematocysts, the molecular assembly is only partially understood. Based on our cloning of minicollagens and NOWA we now analyze the assembly process in a proteom project (2D-gel electrophoresis, peptide mass fingerprinting, and tandem-MS). In parallel we generate antibodies and GFP-fusion constructs to analyze the assembly process. |