Current Research
Development of immunotherapeutic treatment approaches
Nowadays antitumor vaccinations are regarded as promising treatment strategies. However, our knowledge about the immunogenic structures in gliomas and how to activate the patient’s own immune system is still poor. Therefore some major goals of our group are:
• to test feasibility of vaccination therapies in glioma patients, especially in the context of the blood-brain-barrier (Steiner et al., JCO, 2004)
• the identification of immunogenic tumor-associated structures that could be used as vaccine antigens (Beckhove et al., J Clin Invest, 2010)
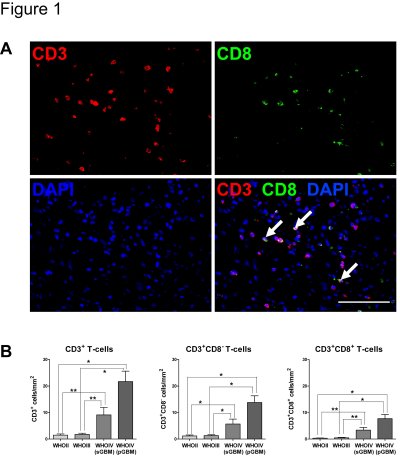
• to learn more about the relevance of immune infiltrates for spontaneous immune responses and patient outcome (Lohr et al., Clin Cancer Res., 2011, Fig.1)
 |
|
Fig. 1: T-cell infiltration increases with malignancy (Lohr et al., Clin Cancer Res., 2011)
(A) Representative epifluorescence images and overlay of pGBM tissue stained for CD3 (red) and CD8 (green) and DAPI (blue). Arrows identify cytotoxic T-cells (CD3+CD8+). Scale bar: 100µm. (B) Computational quantification of T-cell infiltration among gliomas of different WHO grade (n=93, Study Sample A). Data are expressed as mean +-SEM. Asterisks label significant differences (**p<0.01; *p<0.05).
|
Characterization and treatment of glioma stem cells
Recent evidence suggests that the clinical properties of gliomas are largely determined by a small subpopulation of cancer cells that can continuously self-renew and regenerate the tumor. To learn more about these cells our specific aims are:
• to analyze the biological and clinical relevance of glioma stem cells (Zeppernick et al. Clin Cancer Res, 2008)
• to learn more about differentiation and resistance towards physiological differentiation in glioma stem cells (Campos et al., Clin Cancer Res 2010, Am J Pathol 2011, Glia 2011)
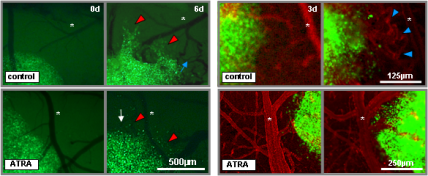
• to characterize their therapy resistance in more detail (Campos et al., Clin Cancer Res 2010, Fig.2)
One of the most powerful tools to achieve these goals is a collection of frozen brain tumors that were operated in the department over the last 15 years, a collection of corresponding blood sera, and a patient data bank including relevant clinical parameters, further treatment and outcome. These resources are complemented by corresponding primary cultures and tumor stem cell lines from many of these patients, which together permit functional analyses and preclinical in vivo studies.
 |
|
Fig. 2: ATRA induces anti-migratory, growth-inhibiting and anti-angiogenic
effects on glioma stem cells in an in vivo therapeutic setting (Campos et al., Clin Cancer Res., 2010) Left panels: Intravital fluorescence microscopy images showing migratory activity of GFP-transfected glioma stem cell spheroids treated with ATRA (lower panel) or vehicle alone (upper panel) at day 0 and day 9 (red arrowheads label migrating cells, white arrow indicates tumor size decrement, blue arrow points at tortuous vessels forming in close vicinity to the tumor, asterisks label reference vessels). Right panels: Intravital fluorescence microscopy images showing vessel activity in the proximity of GFP-transfected glioma stem cell spheroids treated with ATRA (lower panel) or vehicle alone (upper panel) at day 3 and day 6 with vessels counterstained with rhodamine-conjugated dextran (blue arrowheads indicate tortuous, newly formed tumor capillaries, asterisks label reference vessels). |

