Current Research
Sensory-controlled, goal-directed motor sequences are crucial for the survival of an animal. The research group ‘Neural circuits and behavior’ aims at identifying fundamental neural mechanisms that enable an animal to rapidly analyze its sensory environment, to make behavioral decisions and to generate complex motor sequences. To achieve these goals, we use a combination of modern technologies in the zebrafish model system, including high-speed kinematic analysis of swimming behavior, multi-photon calcium imaging, electrophysiology and closed-loop visual stimulation methods. For functional circuit analysis, we employ genetically encoded reporters of cellular activity. Another focus is on the analysis of the central nervous system at the connectomic level, using serial block-face electron microscopy.
|
 |
|
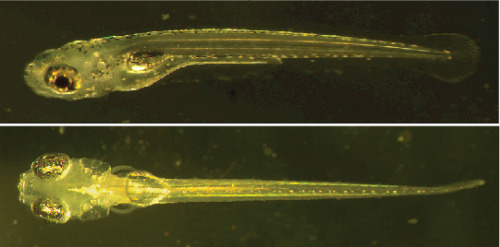
Fig. 1: Zebrafish larvae
Top: Wild-type zebrafish, six days post-fertilization. Bottom: Mutant zebrafish (nacre), lacking melanophores, six days post-fertilization.
|
Virtual prey capture sequences in a closed-loop visual environment
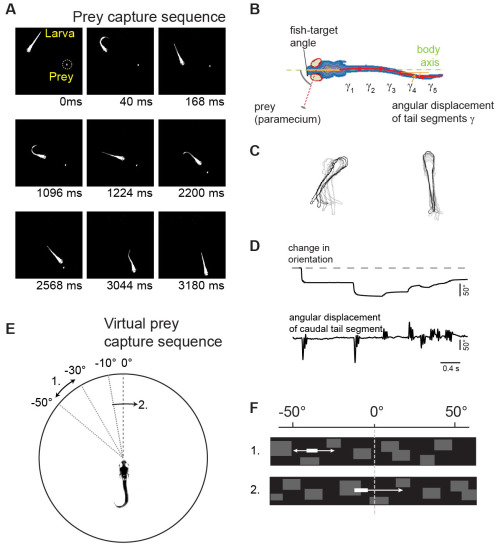 |
| Fig. 2: Virtual prey capture assay (A) Single frames of a prey capture sequence. (B) Measurement of eye and tail movements. (C) Kinematic analysis of orienting swims (left) and forward swims (right). (D) Time course of fish orientation and tail movements during a prey capture sequence. (E) Virtual prey capture behavior: larva partially embedded in center of arena. Stimulus location and direction indicated by curved arrows. (F) The virtual prey as seen by the larva. |
Layer-specific processing of stimulus motion in tectal microcircuits
Neurons sensitive to the direction of a moving stimulus have been observed at all levels of the visual system, but the underlying mechanisms remain controversial, especially in visual centers beyond the retina. A critical question is whether direction selectivity is coded in retinal ganglion cell activity and relayed to downstream targets, or whether direction selectivity emerges de novo in higher visual circuits from basic, topographically organized input maps. We could address this question for two morphologically distinct cell types in the zebrafish optic tectum (Gabriel et al, 2012). Using the Gal4-UAS-expression system, distinct neuronal cell types in the tectum were labeled with the genetically encoded Ca2+ indicator GCaMP-3. Multi-photon Ca2+ imaging showed that cell types with directional preference for opposing directions were morphologically distinct and exhibited dendritic arborizations in different layers of the tectal neuropil. The dendritic profiles colocalized with presynaptic retinal ganglion cell terminals in sublayers with corresponding directional tuning. Furthermore, multi-photon targeted patch clamp recordings show that these cell-types receive directionally tuned excitatory inputs in the preferred direction and weaker, tuned inhibitory inputs. These findings demonstrate a remarkable correspondence between the functional and structural profile of morphologically distinct cell types, which indicates that the central principle of lamina-specific feature extraction applies not only to the retina but also to higher visual centers.
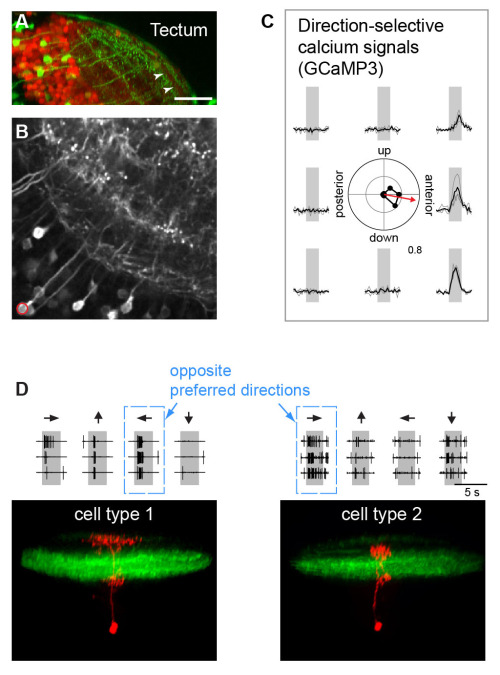 |
| Figure 3: Direction-selective cell types in the optic tectum (A) Expression of GFP in a specific cell type highlights laminar organization of tectal neuropil. (B) Expression of GCaMP3 in the same cell type. (C) Calcium signals exhibit directional selectivity: the neuron marked in B prefers stimulus motion to anterior. (D) Bottom: Two structurally different neurons (type 1 and type 2, red) exhibit opposite preferred directions.Top: Firing rates in response to different stimulus directions (indicated by arrows) reveal directional preference. |
Classification of object size during visually guided prey capture
Zebrafish larvae perform orienting swims towards a prey-like target or away from a threatening object depending on the size of a visual stimulus. Using high-speed kinematic analysis of swimming behavior, multi-photon calcium imaging, in vivo electrophysiology and closed-loop visual stimulation methods, we find that object size is classified in the activity of different neural populations throughout the retinotectal system (Preuss et al., 2014). First, we devised visual stimuli that closely mimic salient visual features of a prey-like object. In response to these ethologically relevant stimuli, we observed that object size is encoded in the activity of different retinal ganglion cell types (RGC) projecting to the optic tectum. Then, using multi-photon targeted patch clamp recordings, we find that a class of horizontal cells called superficial interneurons (SINs) exhibit distinct, size-dependent synaptic inputs, depending on the tectal layer in which their dendrites arborize. SINs fall into different response types, with a distinct class of SINs preferentially tuned to small, prey-like stimuli. These SINs arborize in the most superficial neuropil, where RGCs are predominantly tuned to small moving objects. At the next processing stage, tectal cells in the periventricular zone exhibit differential size selectivities, with approximately equal fractions of cells tuned either to small or large moving objects. We conclude that behaviorally relevant size classification begins in the retina, and that parallel, size-selective channels activate distinct, size-processing subnetworks in the tectum. These distinct subnetworks may represent a bifurcation point for behavioral decision making during visually controlled motor behavior.
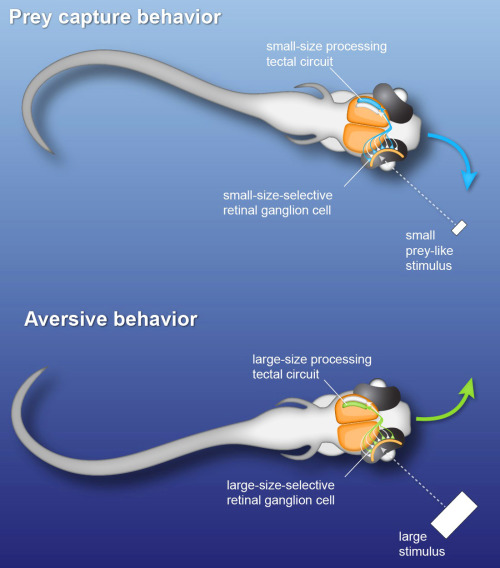 |
| Fig. 4: Fast perceptual decision making in the zebrafish visual system. Small and large objects activate distinct circuits in the visual system of zebrafish larvae. This separation begins in the eye and determines the direction of swimming behavior. |
The wiring diagram of the zebrafish spinal cord
A wiring diagram of the central nervous system (CNS) would represent a valuable structural basis for understanding information processing in this vertebrate model system. In collaboration with W. Denk, we aim to analyze the CNS of the larval zebrafish at the connectomic level, using serial block-face electron microscopy (SBEM). We have acquired a high-contrast SBEM data set comprising several segments of the zebrafish spinal cord, which yields information on how the reticulo-spinal system connects to spinal motor circuits. This allows us to map the synaptic contacts between descending hindbrain neurons, spinal interneurons and different pools of motor neurons in order to address the question whether recruitment patterns of motor neurons may be implemented in specific connectivity rules between these cell types.
Related publications:
Preuss, S.J., Trivedi, C.A., vom Berg-Maurer, C.M., Ryu, S, and Bollmann, J.H. (2014).
Classification of object size in retinotectal microcircuits.
Current Biology 24, 2376-2385.
Trivedi, C. A. and Bollmann, J. H. (2013).
Visually driven chaining of elementary swim patterns into a goal-directed motor sequence: a virtual reality study of zebrafish prey capture.
Frontiers in Neural Circuits 7 (86): 1-18.
Gabriel, J. P., Trivedi, C. A., Maurer, C. M., Ryu, S., and Bollmann, J. H. (2012).
Layer-specific targeting of direction-selective neurons in the zebrafish optic tectum.
Neuron 76 (6): 1147-60.
Bollmann, J. H. and Engert. F. (2009).
Sub-cellular topography of visually driven dendritic activity in the vertebrate visual system.
Neuron. 61 (6): 895-905.
Bollmann, J. H. and Sakmann, B. (2005).
Control of synaptic strength and timing by the release-site Ca2+ signal.
Nat. Neurosci. 8: 426-434.
Related press releases:
1.) http://www.mpg.de/8416636/zebrafish-eye
2.) http://www.mpg.de/7259556/hunting-behaviour-zebrafish-larvae?filter_order=LT&research_topic=BM-NB
3.) http://www.bio-pro.de/magazin/index.html?lang=de&artikelid=/artikel/09567/index.html

