Current Research
Role of voltage gated calcium channels in learning and memory
Our focus is the role of LVGCCs in learning and memory, memory extinction and age dependent memory loss. Since no subunit-specific antagonists or agonists are so far available, genetic tools are necessary to dissect the specific role of Cav1.2 and Cav1.3 in normal and pathologic physiologic functions. We have generated mice with conditional Cav1.3 alleles. By breeding them with mice with tissue-specific expression of tamoxifen-inducible cre recombinase, we are able to study the roles of Cav1.3 in defined tissues at specific time-points, thus allowing for example to differentiate the role of Cav1.3 in developing, adult and aging organs. We have generated and tested forebrain and hippocampus-specific promoters driving the cre recombinase, enabling us to study the role of Cav1.3 in hippocampus-dependent learning and memory. Recently, we were also able to target sub-hippocampal regions. Such genetic modifications allow to study ithe role of Cav1.3 in selected tissues and generate mouse models for the development of tissue targeted pharmacology for the LVGCCs.
Role of MEGAP in mental retardation
Mental retardation is a common condition affecting between 0.3-3% of the population, depending on severity. We generated mice with mutations mimicking recently identified truncations in a novel RhoGAP protein family (MEGAP) found in a patient with severe mental retardation (Endris et all.;2002) We generated mice with stop codon in exon 3 of MEGAP, resulting in premature termination and Megap ablation. Mice with deleted Megap gene develop hydrocephalus and show defects in neuronal development. We carry behavioral and molecular studies of those mice and gene expression profiling in hippocampus and cortex of transgenic mice to identify pathways affected in mental retardation. Our results indicate that ERK1 pathway is significantly downregulated in Megap knockout mice.
Modeling serotonergic defects in depression using conditional regulation of serotonin concentration in transgenic mice.
The serotonergic system is an important modulator of many developmental, behavioral and physiological processes and its perturbation is a major etiological component of depression. The serotonin transporter (5-HTT) plays a key role in the regulation of central serotonergic neurotransmission by removing 5-HT from the synaptic cleft. It is hypothesized that diminished serotonergic neurotransmission predisposes to depression. Stressful life events in humans are known to be followed by depressive episodes in susceptible individuals. Vulnerability seems to be genetically anchored potentially in the serotonergic system. We have generated congenic C57Bl6 mice carrying the tryptophan hydroxylase 2 allele (Tph2) of the DBA mouse strain. Tph2 is a key enzyme in serotonin synthesis, the DBA allele is only about 50% as active as the C57 allele. By combining this defined genetic background with conditional alleles of TPH2 and 5-HTT generated in our laboratory, we are able to manipulate serotonin concentration in wide range in the mouse brain either during development or in adult animals.
Mice with low Tph2 activity show increased anxiety. Currently, we test the interaction of genetic vulnerability generated by conditional manipulation of serotonin concentration with chronic mild stress. This model provides opportunity to study pathways specifically affected in vulnerable animals.
Modeling serotonergic defects in depression using conditional regulation of serotonin concentration in transgenic mice.
The serotonergic system is an important modulator of many developmental, behavioral and physiological processes and its perturbation is a major etiological component of depression. The serotonin transporter (5-HTT) plays a key role in the regulation of central serotonergic neurotransmission by removing 5-HT from the synaptic cleft. It is hypothesized that diminished serotonergic neurotransmission predisposes to depression. Stressful life events in humans are known to be followed by depressive episodes in susceptible individuals. Vulnerability seems to be genetically anchored potentially in the serotonergic system. We have generated congenic C57Bl6 mice carrying the tryptophan hydroxylase 2 allele (Tph2) of the DBA mouse strain. Tph2 is a key enzyme in serotonin synthesis, the DBA allele is only about 50% as active as the C57 allele. By combining this defined genetic background with conditional alleles of TPH2 and 5-HTT generated in our laboratory, we are able to manipulate serotonin concentration in wide range in the mouse brain either during development or in adult animals.
Mice with low Tph2 activity show increased anxiety. Currently, we test the interaction of genetic vulnerability generated by conditional manipulation of serotonin concentration with chronic mild stress. This model provides opportunity to study pathways specifically affected in vulnerable animals.
Inducible and regulated gene expression in the rat brain
The recent progress in molecular neuroscience allows first insights in mechanisms of neuronal function on molecular and cellular level. On the behavioral level, this has been to some degree complemented by studies in genetically modified mice. Largely due to the lack of germline transmissible embryonic stem cells (ES) in rats, large effort to re-establish many of well studied behavioral, pharmacological and electrophysiological rat models in mice was undertaken; so far with only partial success. Many of the complex learning behaviors exhibited by rats are not matched by similar mice behaviors. We therefore developed molecular tools that allow to study the role of genes in complex behaviors in rats:
We have generated transgenic rats with forebrain specific, tetracycline controllable expression of transgenes. Here, we used combination of large genomic BAC DNAs with CaMKII- tTA transgene to target transgene expression to the forebrain and an insulator LC1 locus to obtain reproducible and highly inducible expression of the tetO driven transgene.
In the absence of germline going ES cells, we explore the miRNA technology for generating inducible, brain specific knockdowns in rat brain.
By combining the above methods, we hope to manipulate bi-directionally (overexpression and knockdown) gene expression of rat genes and make rat learning models accessible to reverse genetics.
We have generated transgenic rats with forebrain specific, tetracycline controllable expression of transgenes. Here, we used combination of large genomic BAC DNAs with CaMKII- tTA transgene to target transgene expression to the forebrain and an insulator LC1 locus to obtain reproducible and highly inducible expression of the tetO driven transgene.
In the absence of germline going ES cells, we explore the miRNA technology for generating inducible, brain specific knockdowns in rat brain.
By combining the above methods, we hope to manipulate bi-directionally (overexpression and knockdown) gene expression of rat genes and make rat learning models accessible to reverse genetics.
Age dependent memory loss
Aging is associated with a decline in memory. This impairment is progressive and widespread, affecting about 40% of people over the age of 65. Animal models have been developed to study both the genetic and environmental components of age-dependent memory decline. Interestingly, even in inbred strains of rats or mice only a part of the genetically homogenous population develops memory deficits with aging, indicating that epigenetic changes, involving changes in gene expression, are the molecular keys to the memory decline. We have generated genetic tools which allow us to manipulate concentrations of both NMDAR and LVGCC selectively in the hippocampus of the transgenic rats. We have generated transgenic rats with the NMDAR (NR1) and LVGCC ( α1D) expression controlled reversibly by tetracycline regulated promoter (NMDA tet-O and LVGCC tet-O). To target the receptors expression to the hippocampus, we breed the NMDA tet-O and LVGCC tet-O mice with tTACaMKII rats expressing the tTA activator under control of the CaMKII promoter.
To inducibly and reversibly decrease the NMDAR and LVGCC concentration in the neurons, we have developed a tTA based expression system for overexpresion of miRNAs targeting both receptors. These d genetic tools allow us to perform long-term manipulations of NMDAR and LVGCC in the rat hippocampus. We can thus perform longitudal studies with the same rats over their lifetime and combine behavioral analysis with neuroimaging.
 |
|
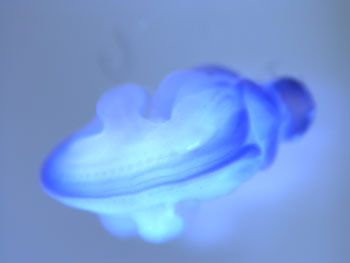
Fig1. Expression of Megap mRNA in mouse embryo (E11). Wholemount in-situ hybridization with antisense Megap RNA probe. At E11, Megap is expressed in the brain and spinal cord.
|
|
|
 |
|
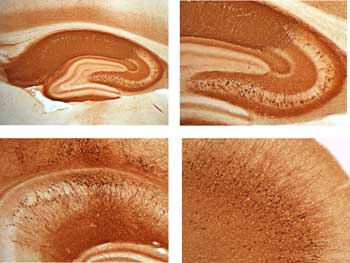
Fig 2. Inducible expression of eGFP in the forebrain of transgenic rats. Rats with the tetracycline activator driven by CamKII promoter CamKII-tTA) were bred to rats with ptet promoter driven eGFP (ptet-eGFP). Double transgenic rats (CanKII-tTA x ptet-eGFP) show high expression of eGFP as detected by anti-GFP antibody. The expression of the reporter is detectable in the forebrain and can be regulated by doxycycline in drinking water.
|
Editor:
A. Summerfield
Latest Revision:
2013-02-26

