Bio(in)organic Chemistry, Molecular Probes
Target-specific molecular probes are exciting tools for the life sciences and hold promise for advancing precision medicine. Research in the Krämer group focuses on the design and synthesis of chemical probes for the recognition and sensitive detection, often by fluorescent signaling, of biomolecular targets. A typical project involves synthesis, characterization and evaluation of function, and may also include the creation for ultrasensitive assays using amplified detection. Group members have the opportunity to become familiar with a wide variety of chemical and biochemical techniques, including the working with enzymes and living cells, often in collaboration with biologists and medical scientists. Examples of our research are given below.
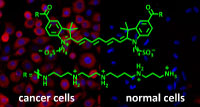
PhD project “Ultrabright fluorescent labels for cancer cell imaging”
Aim of the project is the synthesis of ultrabright small molecule fluorescent labels by attaching several fluorophore entities to a molecular framework. The challenge is to overcome self-quenching of the fluorophores. The project will built upon promising results of a recent master thesis in our group. The best performing labels shall be coupled to a cancer cell selective ligand (e.g. folate, S. König, R. Krämer, Chem. Eur. J., 2017, 23, 1-8.) and applied to the selective fluorescent labelling of cancer cells. Techniques: organic and inorganic synthesis, fluoerescence spectroscopy, cell culture, flow cytometry. Compensation: E13/2. The candidate is expected to supervise undergraduate courses. Start 01.08.2023 or later.
Applications to: kraemer@aci.uni-heidelberg.de
Near-infrared fluorescent probe targets cancer cells
Chemical probes that selectively visualize cancer cells are emerging as powerful tools in clinical cancer management, including early diagnosis, precise surgical resection and monitoring of therapeutic response. The new polyamine-modified indocyanine probe strongly accumulates in live cancer cells with an overactivated polyamine transport system but not in healthy cells (see figure: cancer cells are red, all cells are visualised by blue-stained nuclei. Fluorescence microsopy images. Figure reproduced from S. Koenig, S. Oez, R. Kraemer, Chem. Commun. 2015, 51, 7360 with permission from the Royal Society of Chemistry). Near-infrared fluorescence of the probe overcomes interference by autofluorescence of cells and tissues. A significant advantage over antibody-based markers is the short incubation time, enabling cancer cell identification within a few minutes.
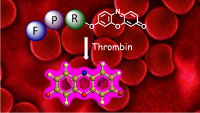
Fluorogenic peptides detect new anticoagulants in whole blood
Direct optical detection of analytes in whole blood is challenging due to hemoglobin interference. PepRes (peptide-resorufin) stands for a new class of fluorogenic, specific protease substrates that combine catalytic signal amplification with intense fluorescence emission at the edge of the “optical transparency window” of blood. The outstanding performance makes PepRes attractive for protease biomarker detection but also enables the quantification of therapeutic protease inhibtors, such as the direct oral anticoagulants (DOACs) which have recently emerged as widely used blockbuster drugs. In spite of increasing awareness of the need for drug monitoring in specific situations, point-of-care tests for measuring blood levels of individual DOACs are currently not available in clinical settings. The PepRes assay selectively detects about 30 ng/mL of the thrombin inhibitor dabigatran in a drop of blood using a portable fluorimeter. D. Arian, J. Harenberg, R. Kraemer, J. Med. Chem., 2016, 59, 7576. Ruprecht Karls Universität Heidelberg, Germany. Chromogenic and fluorogenic peptide substrates for the detection of serine protease activity. Inventors: R. Krämer, D. Arian, J. Harenberg. Publication date 09/02/2017. WO. Patent application WO 2017/021004
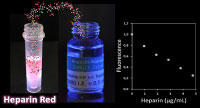
Sensing of sulfated polysaccharides with Heparin Red
Sulfated polysaccharides are complex biomolecules of great biological and medical importance. Examples include the anticoagulant drug heparin (about one billion doses applied annually), heparan sulfate proteoglycans as ubiquitous cell-surface (co) receptors and mediators of endocytosis, and algae-derived fucoidans as bioactive ingredients of functional foods. The detection and analysis of sulfated polysaccharides is challenging due to their inherent structural heterogeneity. Heparin Red is a red-emissive fluorescent probe that enables the direct, sensitive quantification of heparins and other sulfated polysaccharides in various biological matrices by a simple mix-and-read assay. An emerging application of Heparin Red is the pharmacokinetic monitoring of drug candidates in clinical trials, such as chemically modified heparins for anticancer therapy. U. Warttinger, C. Giese, J. Harenberg, E. Holmer, R. Kraemer, Analytical and Bioanalytical Chemistry 2016, 408, 8241.